A question of timing: A lawsuit claims Gilead Sciences could have developed a less-harmful version of its HIV treatment sooner

- Share via
More than a decade ago, researchers at Gilead Sciences thought they had a breakthrough: a new version of the company’s key HIV medicine that was less toxic to kidneys and bones.
Clinical trials of the new compound on HIV-positive patients in Los Angeles and several other cities seemed to support their optimism. Patients needed just a fraction of the dose, creating the chance of far fewer dangerous side effects.
But in 2004, just as the Foster City biotech firm was preparing for a second and larger round of patient studies, Gilead executives stopped the research. The results of the early patient studies would go unpublished for years as the original medication – tenofovir – became one of the world’s most-prescribed drugs for HIV, with $11 billion in annual sales.
More than six years later, though, in 2010, Gilead restarted those trials. The new version of the drug, which the company says is safer, was approved in November under the brand name Genvoya.
The executives’ decisions stand to extend Gilead’s domination of the global HIV medicine market for years. Analysts project the company will reap tens of billions of dollars in sales that otherwise would have vanished with the expiration of tenofovir’s patent in 2018.
That has pleased the company’s investors. But it has stirred criticism among patients and caregivers, and prompted a lawsuit. The critics believe the new, less harmful form of the drug could have been developed sooner – and wasn’t because the company wanted to extend its patent-protected profits.
Today, tenofovir is taken by more than 627,000 Americans, or about 80% of those being treated for HIV, and 9 million more around the world.
------------
FOR THE RECORD
May 29, 8:33 a.m.: An earlier version of this post said Genvoya is taken by more than 627,000 Americans. Tenofovir is taken by that many patients.
------------
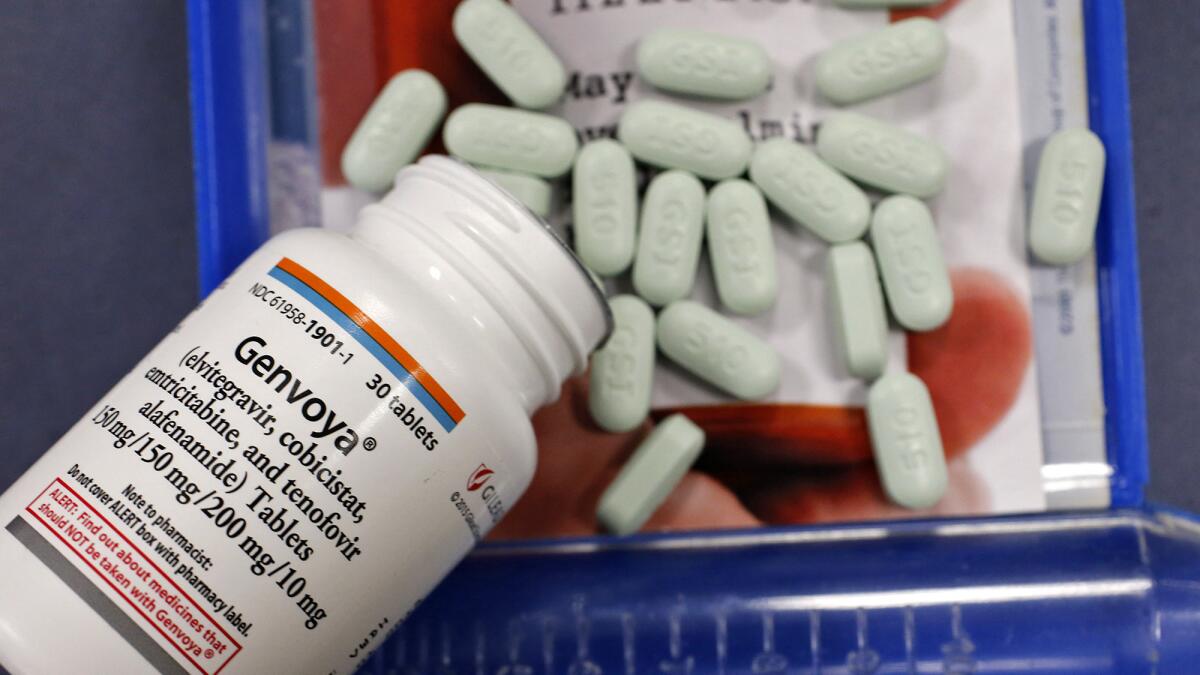
Looking back, Tim Horn of the Treatment Action Group, which advocates for AIDS patients, said: “That’s a decade of potentially avoidable kidney and bone toxicity.”
Horn said Gilead’s decision to resume trials as the original drug’s patent was nearing expiration “suggest that this is much more about market dominance than it was about finite resources for research and development.”
Gilead executives say the drug’s patent expiration had nothing to do with their decision to halt trials in 2004.
“It’s simplistic to look back and say, well, TAF is a safer version and why didn’t you develop it sooner,” said Norbert Bischofberger, the company’s chief scientific officer, using the shorthand name for the new drug.
Bischofberger said Gilead stopped the project to shift money to looking for another type of HIV medicine, known as an integrase inhibitor. The company restarted the research years later, he said, after it saw a need for a less toxic drug for aging HIV patients, who are more susceptible to kidney and bone problems.
“It was then we said, ‘Let’s revisit TAF,’” Bischofberger said.
Protecting prices
With billions of dollars of revenue at stake, pharmaceutical companies are highly motivated to keep their blockbuster brands protected by patents, which prevent generic drugs from flooding the market and driving down prices.
A patent permits a company to exclusively sell a drug for 20 years, giving it time to recover the cost of discovery and make a profit. By modifying a drug’s formula, combining it with other medicines or even changing its dispenser, a company can file for additional patents and extend the period when it can charge premium prices.
Just before the patent expired on AstraZeneca’s blockbuster drug Prilosec, for example, the company tweaked the formula to create Nexium. The company said Nexium was more effective at treating complications of chronic heartburn, though that is a claim some critics dispute. Other companies have created longer acting or controlled-release formulas such as the sleeping pill Ambien CR.
But the high prices of brand-name medicines have put drug companies under intense scrutiny in recent years, and Congress has held hearings to investigate. U.S. drug spending, based on invoice prices to pharmacies, rose 12% last year, according to IMS Health, which sells data to the industry.
Gilead is one of the world’s dominant drug companies today largely because of its success in making tenofovir a cornerstone of HIV treatment. A year of treatment with Gilead’s HIV medicines costs about $30,000, most of it paid by insurers or the government.
The drug is now a component in five of the top six HIV drug regimens recommended by a national panel to fight the human immunodeficiency virus. The World Health Organization considers it among the globe’s essential medicines.
Wall Street analysts had expected the company’s HIV medicine sales to begin falling in 2018 when the patent on the original drug expires. But that forecast changed with the arrival of Gilead’s new pill Genvoya – a combination of TAF and three other medicines.
The compounds in Genvoya are protected by multiple patents, the last of which now expires in 2032 – more than 40 years after tenofovir was invented.
James Krellenstein of ACT UP, an activist group for AIDS patients, believes that the company delayed the development of a less-toxic version of tenofovir to extend its profits. “I think it’s amazingly unethical behavior,” he said.
Earlier this year, the Los Angeles-based AIDS Healthcare Foundation, which operates clinics and pharmacies for AIDS patients, sued Gilead, contending that it delayed the less toxic form of tenofovir to manipulate the patent system and keep prices artificially high.
The foundation, which buys tenofovir-based medicines for many of its 600,000 patients worldwide, called Gilead’s moves “a calculated, anticompetitive maneuver” aimed at keeping lower-cost generics off the market. It is asking the court to toss out the patents on the new drug so that other companies can sell it for less.
Gilead denies the lawsuit’s claims. In stark language contained in a recent court filing, the company’s lawyers said the firm “had no duty to develop, test, seek approval of, or launch its new product on any particular timetable.”
Building a powerhouse
Tenofovir was discovered in the 1980s by European scientists. Gilead, then a small biotech firm, bought the rights to sell it and, in 1997, worked with doctors from UC San Francisco to show it fought HIV by blocking an enzyme the virus needs to multiply.
The original formulation of the drug held little sales potential, however, because it had to be given intravenously.
Gilead scientists modified the chemical composition to create a drug that could be taken orally. The federal Food and Drug Administration approved it under the brand name Viread in October 2001.
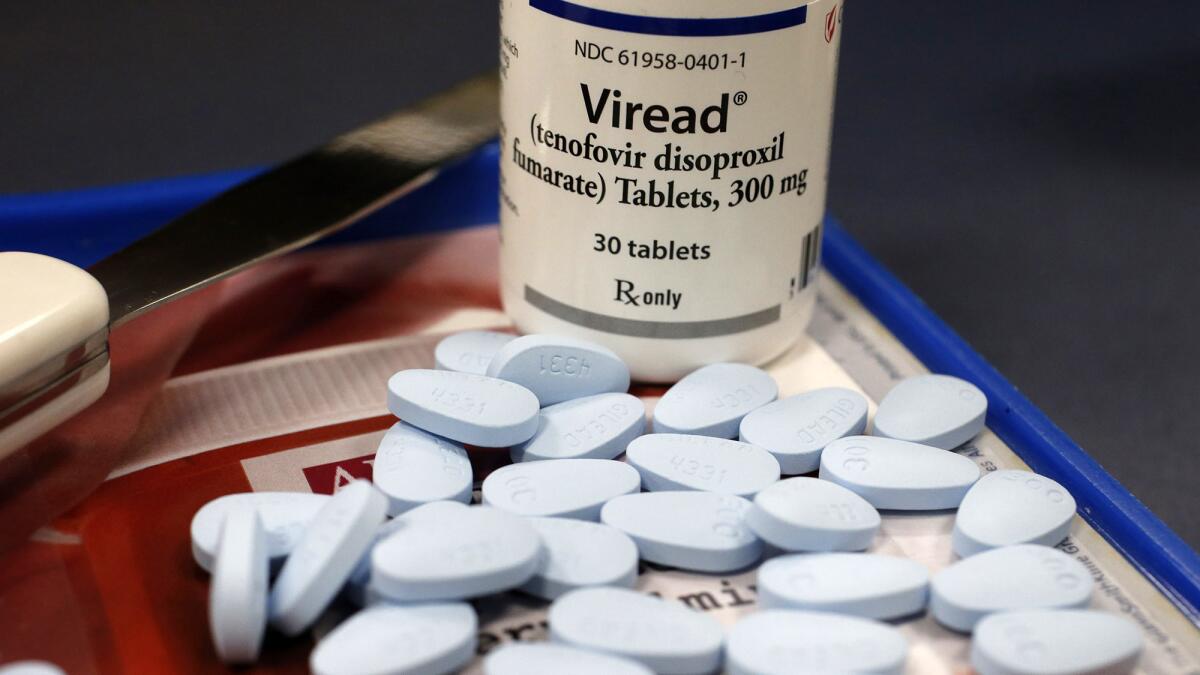
By then, more than a dozen years after the introduction of the first HIV drug AZT, the infection was no longer a death sentence. But with no known cure, patients often took complex regimens involving multiple pills throughout the day.
Gilead built global sales by combining tenofovir with other drugs to create the first once-a-day HIV medicine, a pink oval called Atripla. Today the company sells other combinations of the drugs under the names Truvada, Stribild and Complera.
By 2009, Gilead’s market value exceeded that of pharmaceutical giant Eli Lilly. Nearly 80% of Gilead’s $7 billion in sales that year were from the HIV drugs that included tenofovir.
Doctors’ warnings
Tenofovir had less severe side effects than some HIV medicines on the market, but the company’s early animal studies showed that it could cause damage to the kidneys and bones.
When the drug was approved in 2001, the FDA required Gilead to study whether the medicine would harm humans in the same way.
Two years later, in 2003, the company had received so many reports of patients experiencing kidney failure and other renal problems that it placed a warning on the drug’s label. That label cautioned doctors that a study also had found more bone loss in patients taking tenofivir than another HIV drug.
Several times, U.S. regulators formally warned Gilead that it was downplaying the drug’s risks.
The FDA twice told Gilead that its sales reps had violated the law by giving doctors and patients false and misleading information that didn’t reflect side effects listed on the drug label.
At a medical conference in December 2001, a Gilead salesperson in the company’s promotional booth told attendees that Viread had “no toxicities” and was “extremely safe,” according to the FDA’s 2002 letter. Another Gilead representative called Viread a “miracle drug.”
In a rare move the next year, the agency required the company to retrain its sales reps “due to the significant public health and safety concerns” raised by their repeated false statements, according to the FDA’s July 2003 warning letter.
The reports of kidney failure and other injuries led European drug regulators to ask Gilead in 2006 to remind doctors to monitor patients’ renal function.
In a large study in 2012, doctors at UCSF analyzed a database of more than 10,000 HIV patients at the Department of Veterans Affairs, finding that the risk of chronic kidney disease rose by 33% each year that a patient took the drug.
In response, a Gilead executive told the Wall Street Journal that the study “overstates some of the risks.”
“Gilead was very reluctant to admit the old tenofovir was toxic to the kidney,” said Michael Shlipak, a UCSF doctor who worked on the VA study.
Bischofberger, the company’s science chief, explained in a recent interview that HIV can cause kidney and bone problems, making it hard to blame the drug.
“Just because you observe kidney toxicity while a patient is taking the drug doesn’t prove causality,” he said.
Chronic kidney disease can lead to heart problems and promote dementia. It can also lead to kidney failure, requiring dialysis or a transplant.
“These are young men mostly, often in their 40s,” said Shlipak. “They end up with a quality of life like someone in their 60s.”
Damage to bones can be especially harmful to children, whose frames are still developing.
These are young men mostly, often in their 40s. They end up with a quality of life like someone in their 60s.
— Michael Shlipak, UCSF doctor
Among the drug injury reports sent to the FDA have been a 4-year-old taking tenofovir whose bones suddenly thinned, and a 10-year-old boy who developed leg pain and an abnormal gait. A 12-year-old who was running “felt something snap in his foot.”
Sean Strub, 58, was one of those patients who experienced bone deterioration. He began taking the drug in his 40s and, within a few years, his doctor said he had the bones of “an 85-year-old woman.”
Soon after that, Strub, the founder of POZ, a magazine for those affected by HIV, fell and fractured his ankle in three places, requiring surgery, a metal plate, pins and screws.
“Your bone changes very slowly,” Strub said. “Some people don’t know it is harming them.”
About-face
Gilead scientists were working on a project to reformulate tenofovir and reduce its toxicity even before the original drug was approved by the FDA in 2001.
They selected one reformulation – tenofovir alafenamide fumarate or TAF – for more study because they believed it would more efficiently penetrate cells so that patients would need a lower dose.
In an animal study published in 2001, the company’s scientists found that TAF had 1,000-fold greater activity against HIV than the original medicine invented in Europe. That raised the possibility of less toxicity.
Gilead paid doctors to recruit 30 HIV patients in Los Angeles, New York, Philadelphia and Palo Alto for preliminary studies to see how it worked in humans. The trial found that TAF, the modified formulation, “had greater antiviral potency” at a fraction of the dose.
Despite those promising results, in October 2004, then-Chief Executive John Martin announced that Gilead had stopped the project.
Based on an “internal business review,” he said, executives had concluded the experimental drug was unlikely to be “highly differentiated” from its successful predecessor.
The company’s view changed six years later.
Talking with investors in December 2010, Kevin Young, executive vice president of commercial operations, described “an interesting new molecule.”
But it wasn’t new. It was TAF.
John Milligan, then the company’s president, told analysts that the low-dose alternative could add “a great deal of longevity” to Gilead’s blockbuster product and replace its sales.
And the company began publicly presenting the results from its previous TAF studies.
At a May 2011 medical conference, the company revealed the results of a 2003 study of patients at Stanford and clinics in New York and Chicago. The trial showed the new drug was more effective than the old medicine at one-sixth of the dose. The 2002 study involving Los Angeles patients was published in 2014.
Many medical researchers have long argued that clinical trial results should be published promptly. In fact, a federal law, passed in 2007, now requires that study results be made public no later than 12 months after a trial is completed.
Asked why the company didn’t publish the results earlier, Gilead’s Bischofberger said the company wasn’t interested in publishing them at the time because it was turning its attention to the other HIV research.
Martin Markowitz, a doctor at the Aaron Diamond AIDS Research Center in New York who was hired to recruit patients for both early studies, said he did not remember talking with executives about when the research would be published.
At that time, he added, AIDS scientists were focusing on finding new types of medicines.
“The potential advantages of TAF got put on a back burner,” Markowitz said.
Peter Ruane, a Los Angeles doctor whose name was also on the study, did not respond to several requests for comment.
Today, Gilead’s sales material reminds doctors of the original drug’s toxicity, and reps urge them to prescribe Genvoya and two other TAF-based combination pills approved in the last two months.
To prove its case, the sales force is armed with head-to-head studies – each showing more signs of kidney and bone damage in patients taking the older drug.
Twitter: @melodypetersen
ALSO
Will microbes save agriculture?
North Coast marijuana growers fear a takeover by ‘Big Alcohol’
Senator calls for investigation of Purdue Pharma following Times story on OxyContin
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.











