Stem Cell Hope, Hype
KEVIN MANNIX is a salesman and entrepreneur in the healthcare industry, a husband, father of two and the son of a man who died of a heart attack at 52. In matters of business and of health, he lives by the same principles: Do your research, hedge your bets, avoid regret and -- every once in a while -- take a leap of faith.
Mannix has acted to curb the medical risks he may have inherited from his father. He eats carefully, doesn’t smoke and is a regular at his gym. At 51, his knees creak a little. But he maintains the athletic build that made him a star running back at Penn State and an NFL hopeful who signed briefly with the New York Jets and the Philadelphia Eagles.
Adult stem cells are Mannix’s leap of faith. And after a fair amount of research, he’s intent on putting his in a bank.
“I don’t want to look back in the future and say, ‘Whoops, I should have done that,’ ” he says. “I don’t want to have any regrets about losing this opportunity.”
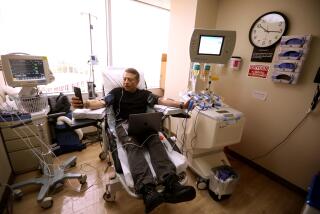
Mannix is betting on the curative powers of adult stem cells -- his own stem cells -- to beat the odds still stacked against him. In the next couple of months, he and his wife Karen plan to travel from their home in Long Island, N.Y., to Encinitas to have stem cells harvested from their blood, then stored for their own future use. It is a medical procedure that could make them either pioneers in a brave new field of healthcare or victims of an industry selling hope beyond the current ability of science and medicine to deliver.
As stem-cell research has gathered momentum in recent years, these microscopic powerhouses have come to spark at least as much hope as they have controversy. And they have spawned new businesses eager to cater to this blossoming of public optimism. Private tissue banks, which offer to harvest and store adult stem cells for a client’s future personal use, are among the most visible of these. And they are springing up across the country.
The result is an industry marked by hype, high cost and only a limited chance that the cells extracted and stored will be of use when the fog of scientific inquiry -- still very much underway -- clears.
These banks draw, in part, upon the excitement generated by embryonic stem cells. But the cells they glean are not the same. Adult stem cells exist in the blood and organs after a human has emerged from the womb, and remain there, hiding in a crowd of more specialized cells. They do not bear the same ethical baggage as their embryonic counterparts, because they can be harvested without creating or destroying new life. But scientists also believe they probably lack the wide-ranging curative potential that embryonic cells have.
Mannix, however, is a believer, and he’s not the only one. In a future that he and many others see as dawning already, adult stem cells will heal the ravages of age, genetic inheritance and environment. They will treat and cure heart disease, cancers, autoimmune conditions such as lupus and rheumatoid arthritis, degenerative diseases such as Parkinson’s and Alzheimer’s and injuries including banged-up bones and joints and damaged spinal cords.
That future may never come, or it may come too late to benefit patients such as Mannix and his wife. It may require stem cells gleaned from embryos, not adult bodies. It may require adult stem cells from different sources than blood, or processed and stored differently than the methods used by banks starting up now.
Nevertheless, private tissue banks are drawing a growing number of customers such as Mannix, who expects to pay $6,000 to harvest his own stem cells from his bloodstream and $400 a year thereafter to keep them cryogenically stored for his future use.
NeoStem, the company that he has chosen to store his stem cells, has launched a $2.5-million plan to expand its services across the country in the next year. It joins a private tissue-banking industry that already includes more than two dozen companies storing the stem cell-rich blood of the umbilical cord harvested at the time of a baby’s birth, one other bank storing stem cells from circulating blood, and an 8-month-old bank that draws and stores stem cells from the soft pulp of children’s baby teeth.
In the coming years, stem cells extracted from fat may further broaden the appeal of private stem-cell banking. At least one publicly traded company -- Cytori Therapeutics of San Diego -- is developing plans to offer plastic surgery patients an opportunity to store these cells after undergoing liposuction procedures. Many others are expected to follow suit.
In short, exhortations to bank one’s stem cells as a form of personal “bioinsurance” seems poised to become the next big thing in consumer health marketing. Those assessing the prospective costs and benefits of doing so will be swimming in scientific waters that are deep, murky and subject to swiftly changing tides. As they navigate their way, they will find scientists and medical professionals coaxing them forward, calling for caution, or swimming to keep up with the entrepreneurial current.
“The technology isn’t ready for prime time,” says Larry A. Couture, vice president of the Center for Biomedicine & Genetics at the City of Hope Cancer Center in Duarte. “Will it be in 10 years? Maybe. But science marches more slowly than people typically appreciate.”
Even those who are enthusiastic about the potential of adult stem cells say they’re dismayed by the pace at which the marketplace is running ahead of the science.
“Everyone gets stuck on what could be the potential of these cells,” says Dr. John Wagner, a University of Minnesota researcher at the forefront of adult stem-cell research. With so much potential and yet so much still unknown about their use in medicine, he asks, “How do you responsibly sell it to the community?
“I’m sort of stuck also,” he says.
*
Cellular transformers
Stem cells are cells that have not yet transformed into a single, defined tissue, be it bone, skin or heart muscle cell. Instead, to at least some extent, they have the potential to become several or many different types of tissue.
Although the most versatile stem cells are thought to be those of the early embryo, scientists now know that various types also exist in adult bodies. There, from birth until death, these cellular transformers stand ready to replenish the blood and immune system and help repair wear and tear on organs damaged by disease or injury.
Scientists have been intrigued by their potential restorative powers since the first ones, from blood, were isolated in 1988. But harnessing them to the task of healing will take a number of technical and scientific leaps. Scientists must find them and figure out what kinds of tissue they can become. They must separate the primitive cells from their surrounding, more mature, tissue, then coax them to grow outside the human body into whatever specialized cells are needed.
And physicians must slip the resulting tissue back into the body without setting off alarms from the immune system, which could destroy the cells or mount an all-out defense that can kill a patient.
Armies of university scientists and research teams from at least 250 U.S., European and Asian firms are working to master each of those steps. They have found and isolated stem cells from several sources (spawning banking proposals each time), and begun to discern what kinds of tissue they can make. The safety and effectiveness of using these cells as treatment are being explored in animal experiments and in several dozen early clinical trials involving humans.
Optimists such as David Harris, professor of immunology at University of Arizona and chief scientific officer at one of the nation’s largest private cord-blood banks, Cord Blood Registry of San Bruno, believe adult stem cells will be widely used in the next three to five years to heal burns, skin ulcers and bone fractures that don’t mend on their own.
The predictions of others are more tempered. Scientists at the forefront of the work acknowledge they have taken only the first steps down a long road toward the goal of regenerative medicine. Growing tissue from stem cells outside the human body is a challenge at which scientists have had virtually no successes, Wagner says. And even efforts to show that adult stem cells can generate a variety of different tissues have fallen short, says Stanford University’s Dr. Irving Weissman, whose success at isolating the adult stem cells in blood gave birth to the field in the late 1980s.
Blood stem cells have so far proven unable to generate any other tissue than red and white blood cells and platelets, Weissman says. And, he adds, there is no evidence that stem cells from fat turn into anything but fat and scar tissues.
“I think people would be buying a pig in a poke, because they have no idea if it’s ever going to work,” he says. “All of the reports that claim that these cells could turn into heart muscle or blood vessels have turned out not to be true -- just not true. “Even researchers with a stake in adult stem-cell banking acknowledge that some of the appeals made to consumers may be getting ahead of themselves.
“Heads are spinning right now,” says Dr. Marc Hedrick, a former UCLA plastic surgeon and president of Cytori Therapeutics, a firm that has pioneered the extraction of stem cells from fat and its possible use in generating tissue for reconstructive surgery and the treatment of heart disease. “I’m bullish on [stem-cell banking] long term. But as a practical matter, either doctors or patients will need better proof of therapeutic use before a big market will develop.”
The big market is out there waiting. The first of the nation’s 78 million baby boomers have turned 60 and are beginning to see a wall of age-related diseases -- Parkinson’s, heart disease, cancers and Alzheimer’s -- bearing down on them. Whether the technology is ready or not, they are receptive to the idea that something they can do now may be able to cure the diseases that killed or disabled their parents.
“I’m not a hypochondriac. I’m a health-conscious person who believes I’d be foolish not to appreciate my family history,” says Mannix, noting that the procedure to draw stem cells from blood is noninvasive and carries little proven risk. “It just makes sense that if I’m going to take steps for a healthy lifestyle, I would take all available steps.”
*
Bone marrow precedent
In discussing the promise of regenerative medicine, many scientists point to the example of bone marrow transplant medicine. For almost three decades, physicians have known that human bone marrow has the power to regenerate the entire blood system, and later discovered the reason why: Marrow contains progenitor cells known as hematopoietic stem cells that can give rise to the various cells of blood and bone marrow. Important for stem-cell banking, hematopoietic stem cells can be coaxed out of the bone marrow to circulate in large numbers in the blood, where they are captured with less discomfort.
These stem cells, found not only in bone marrow but more recently in large concentrations in umbilical cord blood, have become a key part of treatment for leukemia, lymphoma, myeloma and sometimes breast and ovarian cancers. All are commonly treated with high doses of chemotherapy and radiotherapy that can beat back the cancer but wipe out the blood’s disease-fighting white blood cells in the process. Stem-cell “transplants” -- either from one’s own marrow or from the marrow or cord blood of a donor -- are used to rebuild those crucial cells.
This, scientists say, is the most plausible, concrete use that exists today for banking one’s stem cells. But the likelihood of such need is small. In the roulette wheel of life, the chance that a person will need a bone marrow or cord-blood transplant for one of these diseases falls somewhere close to a lifetime probability of being hit by lightning, according to medical studies. Estimates of cord-blood use, for example, range from 1 in 2,700 to 1 in 20,000, with the chance increasing somewhat if one includes the prospect that a family member may use it.
But these days, fewer blood diseases are being treated by transplants, and survival rates from transplants involving unrelated donors are improving. And even when a bone marrow donor cannot be found, marrow increasingly can be cleansed of cancer cells in the lab, enabling patients to use their own marrow even if they’re already sick.
Treatments for some blood diseases are making transplants less likely, and transplants from family members or unrelated donors -- though sometimes hard to find, and imperfect -- are becoming more effective. Yet the “available for a limited-time only” argument is a powerful one for those selling the private banking of cord blood at a baby’s birth. And that “hurry, hurry” push is also a common theme in the marketing of newer stem-cell banks. BioEDEN, the fledgling bank offering the storage of stem cells from baby teeth, argues strongly for the potential usefulness of the stem cells found inside a child’s first set of teeth and stresses that getting these stem cells is easy and presents no risk. (Instead of putting a freshly shed tooth under a child’s pillow, parents are instructed to dispatch it to BioEDEN in a package provided for the purpose.)
But parents are also clearly reminded that this is a source of stem cells with a limited time frame for harvest: These teeth typically fall out between the ages of 5 and 13 -- and when they’re gone, they’re gone.
A lot of BioEDEN’s clients are those who didn’t bank their child’s cord blood and see the chance to bank their baby-tooth stem cells as a “second opportunity,” says company founder and president Jeff Johnson, who says that after eight months in business, BioEDEN already has customers “in the thousands” and is expanding its staff to keep up with demand. (His “tooth fairy storage company” charges $445 for initial collection and an $89 annual maintenance fee.)
Dr. Kari Sakurai, a dentist with a practice in Santa Monica, and an attending staff member of UCLA’s department of hospital dentistry, is a BioEDEN client. She sent her 9-year-old daughter’s last remaining baby-incisor tooth to BioEDEN after reading about the service in a UCLA newsletter. “I knew I had to do something quickly,” she says. “The technology is advancing so quickly. And if you don’t take the opportunity to save something, you’ve lost that opportunity.”
It is harder to argue that early banking of stem cells is urgent for prospective customers such as Mannix. Neither his bone marrow nor fat are going to disappear with age. Even in cases in which stem cells are needed to treat patients whose immune systems have been destroyed by cancer therapy, transplant physicians routinely harvest stem cells well after disease has set in. “There’s not a fire here,” says Dr. Mitchell Cairo of Columbia University, a pediatric oncologist and stem-cell researcher.
Still, those pitching the benefits of stem-cell banking are keen to argue that young, healthy stem cells -- set aside before disease strikes -- would be of better use in a future of regenerative medicine. “It makes sense to do it when you’re healthy,” says Dr. Ron Rothenberg, director of the Healthspan Institute in Encinitas, the first U.S. clinic to offer what it calls a “walk-in stem cell collection and storage center” supported by NeoStem’s services.
Younger cells may well turn out to be better, says Wagner of the University of Minnesota. “But,” he adds, “the bottom line is, there is no data to prove it.”
*
From the lab to the clinic
In the end, says Couture of City of Hope Medical Center, consumers who feel pressed to bank their own stem cells now should temper that urgency with the knowledge that scientists have not come close to delivering stem-cell science to the clinic, and that it will likely be years before it can do so.
Until then, he says, stem cells either from unrelated donors or from one’s own body are available for the few diseases that adult stem cells now are used to treat.
“I would wait 10 years and then start storing your own cells,” he says. “There’ll be greater likelihood of knowing how to use them 25 years down the road. The field is just not as mature as some in the public have perceived from media coverage. It’s just an exciting laboratory thing.”
Yet early stem-cell bankers such as Kevin Mannix may be a crucial force in pushing stem-cell science out of the lab and into doctors’ offices and hospitals, some scientists say.
As banks fill with adult stem cells, researchers will gain more opportunities to study those cells -- to learn how well they survive the freezing process, whether young cells are better than old, what kinds of tissues they really can make and how they can be made to multiply and replicate in the petri dish.
And as their owners become ill with the diseases they dreaded, pioneers such as Mannix will call forth their stem cells, and physicians willing to run such experiments will likely earn more about these cells’ healing properties. “They are pioneering, and once the source exists, I bet it will generate a whole new group of studies that otherwise never would have happened,” Wagner says.
Mannix proudly acknowledges he is a “true believer” when it comes to adult stem cells. But he likes the idea of being a pioneer too. It’s a small risk to take, he reckons, when it comes to a field with such promise.
“We’ve all got plenty of things to do with our time and money. And if I didn’t believe in it, I wouldn’t do it,” he says. “And if I don’t use it, I won’t be moaning.”
*
*
(BEGIN TEXT OF INFOBOX)
Stem cell ABCs
Adult stem cells are different from embryonic stem cells, those hoped-for powerhouses that have attracted political, ethical and religious controversy.
Embryonic cells are called pluripotent because they appear able to become any kind of tissue. They also seem able, if implanted, to adapt themselves to any recipient without prompting an immune reaction, because they have not yet developed an identifying “tissue type” that an immune system could recognize and attack.
Adult stem cells -- the body contains various kinds -- are almost certainly less versatile than embryonic cells. They appear to bear the identifying mark of a “tissue type” and so would likely be rejected by a recipient if he or she is a poor match for his or her tissue type. In contrast to embryonic stem cells, most adult stem cells are called multipotent -- meaning they can become many, but not all, kinds of tissue.
Adult stem cells are found in some organs in greater densities than in others: For instance, the concentration of stem cells in the blood of the umbilical cord is far greater than that in circulating blood. As stem cells are discovered and isolated in different organs, scientists expect they will find that some organs house more multipotent stem cells -- those capable of transforming into a wider range of tissue -- than do others.
Though they may be less powerful than embryonic stem cells, adult stem cells will probably be easier to get and more plentifully available, some scientists say, and as a result, they will likely become a first line of therapy in an era of regenerative medicine.
Either stored in private banks or -- as is done now -- harvested from a patient’s body at the time of diagnosis or injury, these stem cells could allow a patient to “heal himself.” They would bypass the moral dilemma of using embryonic tissue and eliminate the risk that a patient’s body would reject tissue donated by an unrelated stem-cell donor who may not be a perfect match.
-- Melissa Healy
**
Hard sell on cord blood
Do it now, before it’s too late. Over the last decade, private cord-blood banks have sprung up across the country, appealing to expectant parents to seize a “once-in-a lifetime opportunity” to bank a potentially life-saving therapy for their child, siblings or even themselves.
Roughly 500,000 families in the United States have paid fees from $350 to more than $2,000 to place these stem cells in private banks for their own use. The glossy brochures for these services have become ubiquitous in the offices of obstetricians.
This widespread marketing prompted the American Academy of Pediatrics to review the state of stem-cell science and assess the costs and benefits of private cord-blood banking.
The report, published in the January issue of the journal Pediatrics, notes the low probability of a cord blood’s use for childhood disease and raises concerns about the safety of a child using his or her own cord blood in some cases of pediatric cancer.
And it suggests that some of the cord-blood industry’s salesmanship may be exploitative, because “families may be vulnerable to the emotional effects of marketing for cord-blood banking” at the time of a child’s birth.
The Academy encourages the donation of umbilical-cord blood (still treated as medical waste in about 97% of American births) to public stem-cell banks, where it will be available to others. But “private storage of cord blood as ‘biological insurance’ should be discouraged,” it concluded.
The exceptions: cases in which there’s a clear family history of a disease currently treated by stem cells.
“Insurance has to have some definable pay-off,” says Dr. Mitchell Cairo of Columbia University, a pediatric oncologist and stem-cell researcher who was the lead author of the Pediatrics article. “And it’s not there yet in the science. It’s theoretical.”
-- Melissa Healy