Single-Drug AIDS Treatment Rivals Traditional Cocktail in Studies
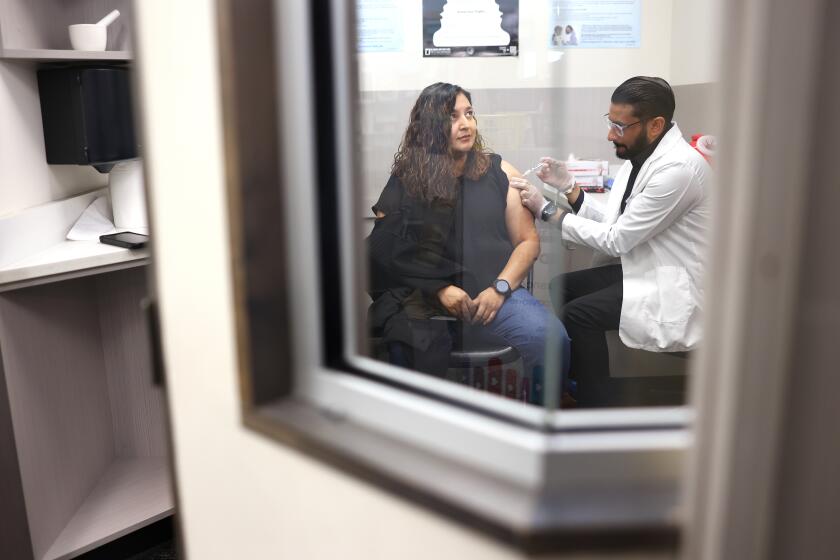
Patients who took a single antiviral drug to combat AIDS fared as well as those on the typical three-drug cocktail, suggesting a simpler and cheaper way of suppressing the virus in the bloodstream, according to several preliminary studies presented Thursday at the International AIDS Conference here.
Three of the studies involved patients who relied on a cocktail of antiretroviral drugs to get the amount of virus under control before slimming down to a single drug from a class known as protease inhibitors. In a fourth study, one group of patients began their treatment with only a single protease inhibitor.
The protease inhibitors were boosted with a compound called ritonavir to increase the drug’s lifespan in the bloodstream.
A simplified regimen could mean lower drug costs and ease some of the side effects from commonly used combination drugs, such as fat redistribution, anemia and kidney problems.
It could also keep more powerful drugs in reserve if a patient stops responding to the first-line therapy, said Dr. Joseph C. Gathe Jr., a Houston infectious diseases expert who was involved with one of the studies.
“Patients expect to live 50 years instead of three years now,” he said. “It’s a marathon instead of a sprint. Why use your best runner at the beginning?”
But many doctors said they were skeptical. If a single drug failed to keep the viral replication under control, the virus could quickly become resistant to it. That fear motivated the development of triple-drug cocktails more than a decade ago.
The single-drug therapy “is potentially viable for the future, but I wouldn’t use it routinely in practice now,” said Dr. Scott Hammer, an infectious diseases specialist at Columbia University Medical Center who led the development of new AIDS treatment guidelines for the International AIDS Society-USA.
Some patients cannot tolerate certain kinds of drugs or don’t want to take them. For that small group, doctors might consider the single-drug therapy with the boosted protease inhibitor.
“These data are reasonable support for that position,” Hammer said.
Combination therapy, pioneered in the mid-1990s, worked so well for so many years that no one thought to change the regimen, said Dr. Susan Swindells, lead author of a simplification study using a protease inhibitor called atazanavir. The study was published this week in the Journal of the American Medical Assn.
“Over time, we realized it wasn’t that simple,” said Swindells, medical director of the HIV Clinic at the University of Nebraska Medical Center in Omaha. “Metabolic complications, for example, take years to develop. It’s kind of like a pendulum shift. People come around to saying, ‘This isn’t so great anymore. Let’s revisit it.’ ”
Swindells’ study followed 34 patients who had taken combination antiviral therapy for at least a year. Switching to atazanavir alone kept the virus in the bloodstream to low levels in 31, or 91%, of the patients.
Genetic tests on the three patients whose viral loads did rise showed no evidence of mutations that would make their viruses resistant to protease inhibitors.
In a study from Spain, 85% of patients who had used combination therapy to suppress HIV were able to keep the virus at undetectable levels for a year by switching to a single protease inhibitor called lopinavir. The regimen, which was taken by 100 patients, worked as well as the triple therapy taken by 98 patients, concluded the researchers.
French researchers tested the efficacy of putting patients on lopinavir alone at the outset of their therapy. Those patients succeeded in lowering their viral loads to undetectable levels at about the same rate as patients who began their treatment with a triple-drug regimen that included lopinavir.
Two of the 83 people taking lopinavir alone developed a virus with a mutation that was resistant to protease inhibitors, while one of the 53 patients on triple therapy developed the mutation, according to the study.
The studies from France and Spain, along with Gathe’s study, received funding from Abbott Laboratories Inc., the company that markets lopinavir under the trade name Kaletra. Swindells’ study was funded by the National Institutes of Health, and Abbott and Bristol-MeyersSquibb provided the medication free.
Many doctors at the conference said they hoped there would be larger studies involving several hundred people that would watch patients for a longer period of time.
“I need more convincing,” said Dr. Chinkholal Thangsing, a physician from India who serves as Asia/Pacific bureau chief for the AIDS Healthcare Foundation, a Los Angeles-based treatment group.