Science / Medicine : Doctors Battling Epidemic Get New Look at Sleeping Sickness : Research: The disease is again raging in Africa. Scientists seek a way to keep the tsetse parasite from tricking the immune system.
A new outbreak of African sleeping sickness in Uganda is providing scientists with a unique opportunity to study the strange parasite responsible for the affliction.
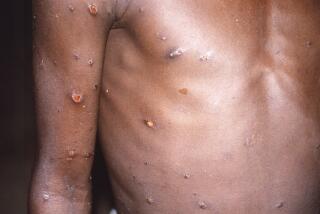
African sleeping sickness is a parasitic disease inflicted on its victims through the bite of the tsetse fly. Untreated, its early symptoms of fever and fatigue give way to apathy, convulsions, coma and inevitably death.
Early stages of the disease are often confused with malaria and late stages with AIDS--both of which are common in Uganda. The two Ugandan epidemics are caused by two variants of the parasite, called trypanosomes.
Trypanosomes have captured the interest of scientists because of their ingenious ability to avoid the host’s immune system. When the tsetse fly bites a human, the trypanosome is injected into the bloodstream.
When the British and other Europeans began in earnest to colonize equatorial Africa in the 19th Century, African sleeping sickness felled native tribesmen, explorers and missionaries alike. The scourge was so severe that all of Central Africa was terrorized. One epidemic, which raged in Uganda early in this century, killed 300,000 people.
Today, according to Dr. Dawson B. Mbulamberi, chief of Uganda’s National Sleeping Sickness Program, a second epidemic is raging uncontrolled in the region bordering Sudan, as well as in Sudan.
During the 1950s the disease was largely controlled in Uganda by spraying DDT and clearing lantana bushes, where the tsetse fly breeds. Since the 1970s, when dictator Idi Amin relaxed controls, the disease has once more begun to appear in southeastern Uganda near where the Nile River joins Lake Victoria.
When Amin was chased into northwestern Uganda in 1979, many residents fled across the border into Sudan, where a different type of sleeping sickness was rampant. This type is more difficult to diagnose and festers for years before eventually killing its victims.
Once a person is bitten and the trypanosome enters the bloodstream, the host’s immune system unleashes antibodies that recognize the distinctive proteins on the parasite’s outer surface. With most types of organisms, the attack by the antibodies would be enough to stop or slow the infection.
Trypanosomes, however, are almost immediately able to change the proteins on their surface so that the first army of antibodies becomes ineffective.
The immune system must then grind out new antibodies designed to attack the “new” organism. A trypanosome has up to 1,000 different surface proteins with which it can challenge the immune system, according to John Boothroyd, a Stanford researcher.
Boothroyd and David Campbell, a UCLA microbiologist, are among the scientists studying how the trypanosome can change its spots by switching on different genes to make a new protein each time it is attacked.
“By sorting out how the parasite’s RNA (ribonucleic acid) is synthesized, how it is used and why this unusual processing is apparently so essential to the organism, we hope to identify a target in the parasite which will respond well to drug treatment,” Campbell said.
The drugs used are exceedingly toxic and must be administered in a hospital. Mbulamberi said 5% of patients die from drug toxicity.