Cancer Patients Using New Drug in Total Remission
With a single treatment, a new drug apparently has caused a remarkable number of complete remissions among patients stricken with a rare form of leukemia, a Scripps Clinic researcher reported Monday.
Reporting on results of 144 patients with hairy cell leukemia, Dr. Lawrence Piro said 85% of those treated with the new drug underwent complete remission of the usually deadly disease. An additional 12% experienced partial remission, and 2% didn’t respond at all to treatment using the drug 2-CdA, or 2-chlorodeoxyadenosine. The remaining patients died of unrelated causes.
The patients have been monitored for signs of the disease anywhere from 14 to 78 months--or a median of 2 years--after being treated with one continuous dose over seven days, Piro said during the American Society of Clinical Oncology meeting in San Diego.
“Any time you have a treatment where one infusion is able to induce a high rate of remission, it becomes very desirable,” said Piro, director of the Green Cancer Center at Scripps Clinic & Research Foundation.
“Have we cured these patients? Only time will tell us.”
Piro first reported success with 2-CdA two years ago in the New England Journal of Medicine after a trial involving 12 patients. The results this week represent the largest trial of the drug.
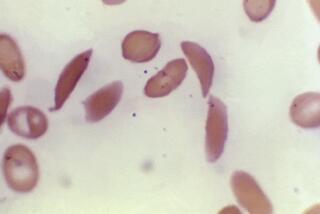
Hairy cell leukemia is a relatively rare but fatal malignancy affecting the blood and bone marrow. As the disease develops, abnormal lymphocytes--or white blood cells--begin growing uncontrollably. Eventually, they take over the bone marrow, wiping out its ability to make clotting and infection-fighting cells. The disease earned its name because the cancer cells look “hairy” when examined under a microscope.
The leukemia afflicts about 6,000 Americans, with 500 to 600 new cases each year. The drug interferon traditionally has been used to treat the disease, but doctors have begun to suspect its shortcomings.
According to data presented here Monday, two drugs--2-CdA and deoxycoformysin, or DCF--appear more effective than interferon in battling hairy cell leukemia. The drug 2-CdA is now under U.S. Food and Drug Administration review and not widely available. DCF is available as a second line of defense when interferon fails.
National Cancer Institute investigators at the conference Monday presented data showing that DCF caused complete remission in 68% of 152 treated patients. In the same study, the remission rate was 11% among the 153 patients treated with interferon, said Dr. Michael R. Grever, the NCI’s associate director of developmental therapeutics program.
In the trial, patients were randomly assigned to receive DCF or interferon. But they were allowed to switch if they failed to respond to therapy. Of the 153 patients who initially received interferon, 101 later switched to DCF. And eight of the 152 patients who initially received DCF later changed over to interferon.
DCF, as well as 2-CdA, may compromise the body’s ability to ward off infections, Grever said. For patients with active infections, it may be more appropriate to treat with interferon until the infection subsides, he said.
In the NCI study, patients were followed a median of 38 months after being treated six months to a year with DCF, also known as pentostatin, Grever said.
“The disease doesn’t go away on its own. I don’t think there’s any question that the drugs are causing complete remission,” Grever said. “We have two very good drugs (2-CdA and DCF). . . . Whether or not we’ve licked hairy cell leukemia is going to take time to see how well these drugs do long-term.”
The question about whether either drug represents a complete cure remains unanswered because the disease itself can be slow to develop. About 10% of those afflicted with hairy cell leukemia live a decade without needing intervention, Grever said. Others get sick within a few years.
But Piro and his colleagues are clearly encouraged by their 2-CdA findings. These results were so promising that Piro and his team decided to try the 2-CdA on other cancers, such as brain cancer. The NCI also will sponsor trials to investigate other applications of 2-CdA.
Piro touts the drug as appealing because it is not only effective but it also lacks side effects, such as nausea and vomiting, that can accompany DCF as well as standard chemotherapy.
So far, fever is the most common side effect researchers have found among 2-CdA patients. About 43% suffered fevers of 101 degrees. Piro believes the cells are so rapidly killed by the drug that they release cytokines, a naturally occurring substance that regulates cells’ growth and can promote fevers.
An additional 10% experienced infections, but Piro maintained that these were caused by the catheters, inserted to allow the patient to undergo continuous 24-hour-a-day medication while at home.
Though Piro on Monday reported his findings on 144 patients, he said he had treated about 350 patients.
“Both drugs--2-CdA and DCF--are excellent drugs,” Grever said. “It’s a major advance. The ultimate decision between one drug and the other is a decision the doctor and patient are going to have to make.”
Last year, patients from 42 states and seven countries flocked to Scripps seeking the 2-CdA treatment.
Cheryl Nichols, a 46-year-old graduate student from Mission Viejo, came to Scripps when she realized that she was finding it difficult to concentrate on her studies. Her concentration had plummeted--forcing her to drop courses.
At first, she tried to dismiss the pounding headaches and the fatigue she felt.
“I was married, a mother, holding down a part-time social work job, and trying to finish my master’s degree,” she said in a prepared statement released Monday. “No wonder I was tired.”
But her doctor diagnosed hairy cell leukemia, and Nichols obtained treatment at Scripps in December. This spring, her cancer in remission, she returned to school, where she will graduate this month “thanks to 2-CdA, “ she said.