Scientists Try Unfertilized Egg as Source of Stem Cells
WASHINGTON — As the nation grapples with the use of human embryos in stem cell research, scientists in Los Angeles and Massachusetts are about to bring a new twist to the debate. They are working on ways to coax unfertilized eggs to grow into embryos that produce stem cells--the very cells that have raised so much hope for their potential to cure disease.
If successful, these researchers would create a human embryo without sperm, and, therefore, without conception--that landmark often cited as the start of human life. Would this embryo be considered a human being? Would destroying it be immoral? Even groups devoted to protecting human embryos are stumped.
“We’re just going to have to research it,” said Laura Echevarria, spokeswoman for the National Right to Life Committee. ‘We don’t know yet what to say.”
The work, which is still in progress and has not yet appeared in scientific journals, is the latest example of how the stem cell debate has come to raise a far larger issue. It is forcing the nation to reconsider what moral value it assigns to human embryos, some of which are being created in wholly new ways.
Researchers believe embryos produced without sperm would self-destruct after several weeks and be unable to grow into a child.
“People are going to have to take a hard look at their beliefs,” said Paul Berg, a Nobel Prize winner and professor emeritus in biology at Stanford University. “If you believe that at conception you’ve created a human being, how does that fit with all these other ways to create embryos?”
Once, the debate over embryonic life was fairly straightforward. Generally, people either supported a woman’s right to control her own pregnancy or they sought to protect life in the womb. Due largely to the Supreme Court’s decision in Roe vs. Wade, they thought of a life’s beginnings in terms of long blocks of time known as trimesters.
Now the stem cell debate is drawing attention to the early life of the human embryo and forcing people to draw distinctions among embryos only days apart in age.
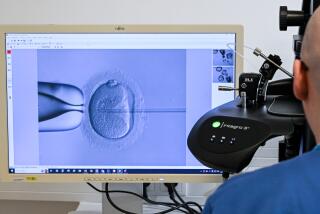
The issue arises because of stem cells, which appear in embryos that are about 5 days old. These cells have the ability to grow in unlimited numbers and to transform themselves into many other cell types--nerve, bone, muscle and more. Scientists hope someday to fashion stem cells into replacement parts for patients, giving new brain cells to Parkinson’s patients, pancreas cells to diabetics and neurons to victims of spinal cord injuries.
Researchers have been using private money to work with stem cells, but after months of deliberation, President Bush announced Thursday that the government, the largest supporter of U.S. medical research, could also fund the work, with a key restriction. He said federal tax dollars could go to experiments using stem cells that had already been extracted from human embryos but not research that required any new embryos to be destroyed.
In making his decision, Bush said he was guided by his belief that an embryo is a human life as soon as egg meets sperm, an event that is often but not universally called conception.
“I think life begins at conception . . . and that influenced my decision, of course,” the president told ABC News on Friday.
But others say that an embryo is only potential human life in its earliest days and that there is no guarantee it can mature into a child. They say a better landmark for determining life is Day 8 or Day 9, when the embryo implants in the uterine wall and has a greater chance of growing to full term.
Under that view, taking stem cells from a 5-day-old embryo poses much less of an ethical dilemma.
Still others say an embryo cannot be considered human life until Day 14, when it develops a “primitive streak,” the beginnings of a backbone. Until that point, a single embryo can divide into identical twins or two embryos can merge into one.
“It is very clear that you cannot speak of a human individual in the first 14 days of development,” said Ronald Green, a Dartmouth College bioethicist. “How can one speak of the presence of an individual soul if the embryo can be split into two or three?”
In addition to the age of an embryo, stem cell research is prompting some lawmakers and ethicists to draw moral distinctions among embryos created in different ways. These include in vitro fertilization, the process--which dates back 23 years--of merging sperm and egg in a laboratory dish to produce “test-tube babies.”
Sen. Gordon Smith (R-Ore.), an abortion opponent, argues, for example, that life begins at conception--but not if that conception occurs outside a woman’s body. “I believe that life begins in a mother’s womb, not in a scientist’s laboratory,” Smith said.
That view allows Smith to support research on embryonic stem cells. Nearly all of the cells are taken from embryos created outside the womb at fertility clinics by patients seeking help in having a child. These patients often create more embryos than they will use, and some have donated their extras to stem cell researchers.
Now researchers in Los Angeles and Massachusetts are adding new wrinkles to the debate by trying to produce human embryos from eggs alone.
Scientists have known for nearly a century that an egg cell, when given the right chemical or electrical stimulus, will begin dividing into an embryo on its own. The phenomenon, known as parthenogenesis, was first discovered in sea urchins, and it led to a small outbreak of hysteria. Surmising that something in seawater caused the urchin eggs to grow, newspapers warned unmarried women not to swim in the ocean, while couples who hoped to have children hurried to the beach.
In mice, parthenogenesis has successfully produced embryos that matured long enough to grow stem cells, said Michael West, chief executive of Advanced Cell Technology Inc., a Massachusetts firm that is working with the technique. But the mouse embryos do not go on to produce baby mice. Though they have no scientific confirmation, researchers assume the same would happen in humans because mice are organisms that mimic the human body in many ways.
“I think that simplifies the moral situation tremendously,” said Jerry L. Hall, a Los Angeles researcher who is also experimenting with parthenogenesis. “Because this embryo lacks the potential to become a child, that implies that it doesn’t have the same status as a normal embryo created by the union of sperm and egg.”
As a result, there are fewer moral implications in destroying this type of embryo for its stem cells, said Hall, who works with the Institute for Reproductive Medicine and Genetic Testing, an affiliate of the Tyler Medical Clinic in Los Angeles.
It is unclear how much progress either team has made because scientific journals bar researchers from discussing their results before publication. West, in fact, would not confirm that Advanced Cell is working with the technique.
But he said: “Hypothetically, could you do this in humans? I’m optimistic that you can.”
Richard Doerflinger, an official of the U.S. Conference of Catholic Bishops, said it was unclear whether the Roman Catholic Church would view parthenogenically grown embryos as a form of human life that deserves protection. However, he said, “we would want to give the benefit of the doubt to the embryo that, yes, it is life.”
Doerflinger also said that, unlike Hall, the church would not base its view on whether the embryo had the potential to grow into a child. “That it might have a short life does not tell us much. There are embryos that die early on that were still human organisms until they died, and they deserve protection while they are alive.”
Yet another technique--cloning--raises separate ethical questions. Some researchers, including those at Advanced Cell, want to use cloning to bypass the problem of tissue rejection, a common complication during organ transplants.
By making a cloned embryo of a patient, they say, scientists may be able to grow stem cells and then replacement tissues that match the patient’s body perfectly, preventing rejection.
But some cloning techniques raise more ethical questions than others. The House of Representatives last month voted for legislation to bar the production of cloned human embryos, both as a medical tool and as a step to create children. Bush said he supports the bill.
But West, in testimony before a Senate committee this month, raised the prospect of a different type of cloning. Using proteins found in egg cells, he said, it may be possible to prompt adult cells, such as skin cells, to revert into stem cells similar to those in embryos.
“They may be embryonic in the sense that they express the genes of the early embryo,” West told the Senate, but these cells “would not have the architecture of an embryo and would not create a pregnancy if put into a uterus.”
For abortion opponents, who are used to thinking of conception as the start of life, these and other techniques are forcing a reconsideration of long-held beliefs.
“We used to say we were devoted to protecting life from conception through natural death,” said Echevarria. “Now we are starting to say ‘creation’ through natural death. You’ve now got so many ways of developing life.”