Bird flu is coming for humans. We can either get ready or court disaster

- Share via
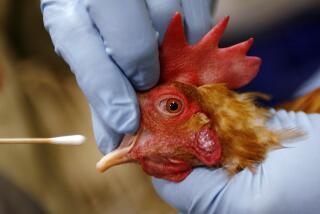
Bird flu has seen a resurgence in the U.S. this year, with California leading the pack. Since the Department of Agriculture detected the virus in dairy herds in March, more than 50 people have tested positive for it, with 34 reported cases in California alone. Last month, a Californian became the first child in the U.S. confirmed to have the virus.
In the nearly three decades since H5N1 was first isolated in commercial geese in Guangdong, China, likely spread from migratory wild birds, it has infected more than 890 people — and killed more than 460 — in 24 countries. Since 2022, millions of egg-laying chickens have been exposed to, infected with and culled or killed by H5N1 in the U.S. What stands out this year is how widespread the infection has been among dairy cows, which are spreading it to farmworkers — the group that accounts for most of the human cases so far.
The virus has been kept somewhat at bay. Although it has mutated to infect humans and about 50 other types of mammals, like an ill-fitting key it still faces challenges to entering human bodies. People have contracted it primarily from direct contact with infected animals, for example by getting milk on their hands at a farm and then touching their eyes, so the case numbers remain modest and disease symptoms in the U.S. generally mild.
State agriculture officials announced a raw milk recall from Stanislaus County producer Valley Milk Simply Bottled, YubaNet has reported.
But as we have seen with other influenza strains, the virus continues to evolve. There have been reports of humans being infected without a clear animal contact. A research paper this month reported that H5N1 is now just one mutation away from attaching more easily to human cells, possibly enabling sustained human-to-human transmission — which could mean more people getting infected and becoming seriously ill, disrupting school, work and our everyday lives.
There is no guarantee that a major human outbreak or pandemic will happen soon. Finding a single mutation in a lab that can facilitate more human infections does not guarantee that this threat will play out in the real world.
But the more transmissions that occur, as is happening now among poultry and dairy cows in the U.S., the higher the likelihood that some of these mutations will appear by chance and take off. A teenager in Canada’s British Columbia with a mutated form of H5N1 became critically ill; such mutations could lead to more streamlined entry into human airways, making people sicker. It is also significant that H5N1 has now infected at least one U.S. pig. Pigs, which contain receptors for both avian and human influenzas, can get simultaneously infected with both, exchange genes and create a novel strain that can more easily infect humans. This is what likely happened with the 1918 flu pandemic and again with swine flu in 2009.
In addition, there have been four influenza pandemics since the early 20th century, following the 1918 pandemic that killed an estimated 50 million people. All of them had origins in avian influenza.
So it increasingly looks like the question is not whether H5N1 will cause a widespread outbreak in humans, but when. The consequences could be severe: As was the case during the early days of COVID, our immune system is not experienced with fighting this novel pathogen, and it increases the chance of more serious disease such as pneumonia and cardiac and brain complications for all ages. Although human cases in the U.S. have been relatively mild, about half of the people who have contracted H5N1 globally have died.
Wild birds get the blame, but the largely unregulated transport of cattle across the U.S. poses too-often-ignored disease risks.
At this crossroads, the U.S. can choose either of two scenarios.
The first is that we do everything right. We could continue to improve the timely surveillance of animals and raw milk — batches of which have been recalled for potential bird flu risk — to better identify infected herds and understand the true magnitude and dynamics of infection. This step will allow us to prepare for an emergency outbreak and know whether any of our interventions, such as employee vaccination programs or biosecurity measures to isolate animals, are working. The Department of Agriculture recently announced that it will test raw milk samples in six states, some of which have identified infected dairy herds and some of which have not. This could provide a glimpse of how extensive the outbreak really is. More states should be included in monitoring, and we still need to test farmworkers and their close contacts more systematically. Hospitals and clinics should move to centralize and share H5N1 data.
Our federal government can prioritize and incentivize scientific discovery of rapid diagnostics, new vaccines and therapeutics. The current supply of H5N1 vaccines is small, but the government can expand its production and consider offering them to farmworkers who request them. We then need to develop a playbook for implementing and disseminating these technologies to the most at-risk populations locally and globally. If we’ve learned anything from COVID, elected officials, federal agencies, public health and healthcare workers have to deliver clear and consistent messaging with empathy. If we take those steps, a major bird flu outbreak does not have to become another pandemic.
The other option is to go the direction the incoming federal government seems poised to support: Shrink public health dollars and expertise at the Centers for Disease Control and Prevention and other federal entities, cut investments in infectious disease research, stymie the use of evidence-based vaccines, therapeutics and diagnostic tests, and support drinking raw milk.
The choice is ours, and only one route promises more suffering for our families and loved ones.
Peter Chin-Hong is a professor of medicine and an infectious disease specialist at UC San Francisco. @PCH_SF
More to Read
A cure for the common opinion
Get thought-provoking perspectives with our weekly newsletter.
You may occasionally receive promotional content from the Los Angeles Times.