Four things Americans should know about Dr. Scott Gottlieb, the new head of the FDA

- Share via
America, you have a new commissioner at the Food and Drug Administration.
Dr. Scott Gottlieb, a 44-year-old physician, was confirmed by the
Here are four things you’ll want to know about Gottlieb.
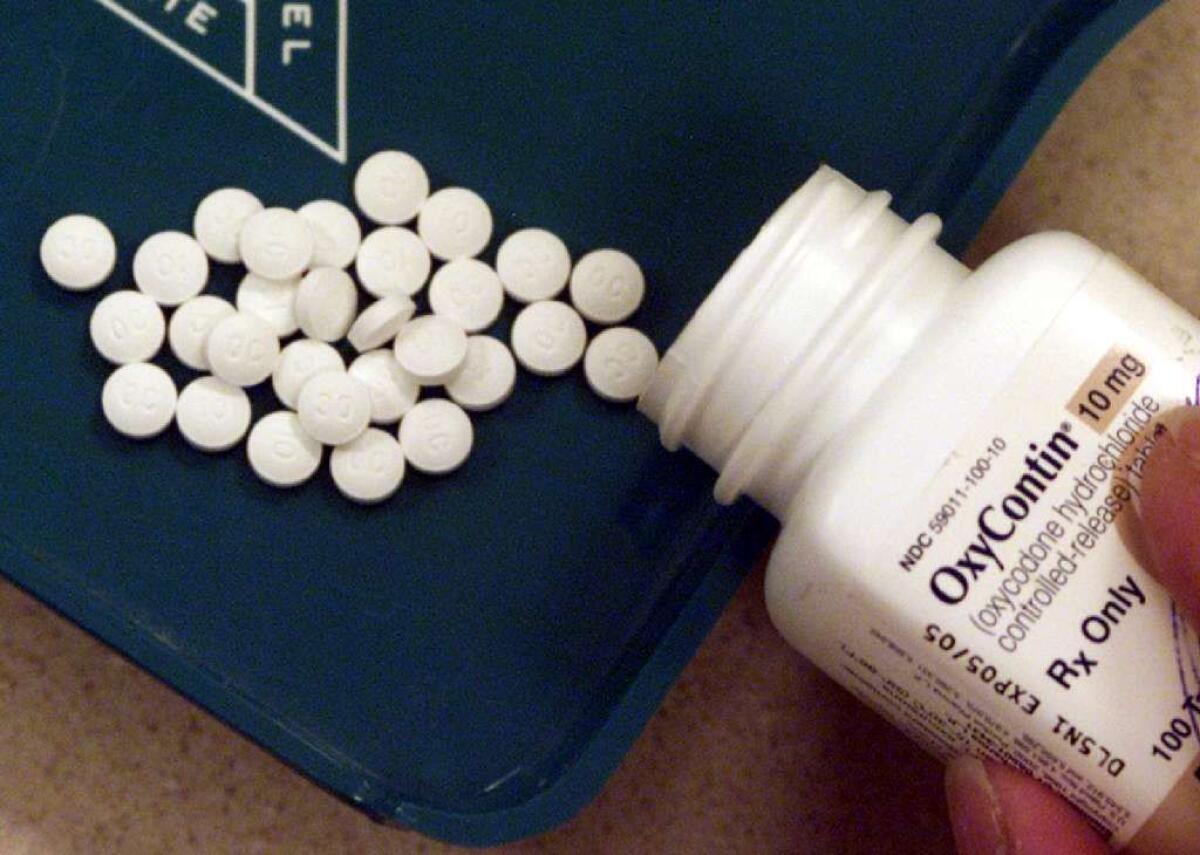
His first priority is to address opioid drug use in the United States, but there’s only so much he can do.
In his Senate confirmation hearings in early April, Gottlieb told senators that the epidemic of opiate drug abuse in the United States "is a public health emergency on the order of Ebola and Zika." On average, 91 Americans die each day of an opioid overdose.
But the FDA’s power to reduce opioid drug use is limited.
The agency’s past decisions have clearly contributed to overdose deaths. The FDA does not regulate physicians’ prescribing of drugs — an important contributor to Americans’ widespread opioid drug use (and subsequent addiction). All it can do is enforce legal limits on the marketing of drugs once they’ve been approved — strictures that drug manufacturers, in their bid to build their markets, routinely flout.
The FDA also reviews new pain medications and new formulations of opioid-based drugs for the U.S. market. In considering whether to approve them, the agency walks a fine line between keeping pain medication available to those who need it and not creating new addicts. Some of the abuse-resistant formulations of opioid pain relievers approved for the market in recent years have driven many addicts to cheaper, more readily available street drugs such as heroin.
He wants to bring prescription drugs to the market faster. Here’s how he proposes to do it.
In his April confirmation hearings, Gottlieb sought to defuse concerns that speeding up the drug approval process would allow less-safe medications to come to market. He called it a “false dichotomy that it all boils down to a choice between speed and safety.”
He repeatedly called the FDA’s review process “the gold standard.” And he stopped short of endorsing proposals that had been floated by other candidates for the job, including plans to approve drugs after they had been tested for safety but before their effectiveness had been established.
But he also said the FDA should “lean forward” to modernize and speed the approvals process.
Gottlieb has long been a fan of what are known in the clinical-trial business as “adaptive trials.” Such human experiments, which are already in limited use, depart from what many scientists define as the “gold standard” in that their design, their study populations, and even their objectives can be altered along the way in response to early results.
A drug company running a clinical trial might be allowed to increase or decrease the number of subjects assigned to different “arms” of a trial, or change who gets assigned to those arms, or how those assignments are made.
The idea is that researchers could shift a clinical trial’s focus in response to early signs of a drug’s strength in, for instance, treating one population (people with very early Alzheimer’s disease, for instance) over another (say, those whose
Rather than conducting a lengthy and expensive series of separate trials to discover which patients might benefit most from a drug, a single adaptive trial could come to the same conclusion after only one study that was allowed to “flex” along the way.
He can play a role in bringing the cost of prescription drugs down, but it’s limited.
Gottlieb has been critical of
In a March 2016 article in Forbes, Gottlieb wrote that while Trump’s plan is “perhaps good politics,” it will “offer consumers little relief.” Drug manufacturers would be unlikely to cooperate because increasing their output of production lines abroad would undermine their own interests. And it’s just as unlikely that other countries would stand for having their medications “skimmed off, only to create local shortages of important medicines,” he wrote.
In its central mission of evaluating the safety and effectiveness of drugs and granting companies the right to market them in the United States, the FDA is not permitted to take price into consideration. But the FDA’s power over the approval of generic drugs is a key lever for bringing down the cost of drugs, and Gottlieb has said he wants to see improvements there.
The company that makes a drug has exclusive rights to the U.S. market for three to seven years after it is granted FDA approval. But once that protection ends, any drug company can seek FDA approval to market a generic version by proving it can manufacture the active drug ingredient and package it for human use safely and reliably. The result: competition that typically drives drug prices down steeply.
So driving down the cost of many prescription drugs will require the FDA’s Office of Generic Drugs to be staffed adequately to speed approval of drug applications. That’s a tall order against the backdrop of the Trump administration’s proposed budget cuts to the Department of Health and Human Services, the FDA’s parent agency.
Also, many drugs — including a broad new category of drugs known as “biologics” — are less simple to copy. The FDA’s cautious approach to approving generic versions of many of these drugs — widely used to treat cancers and autoimmune diseases — threatens to create “monopolies in perpetuity,” Gottlieb has said. If prescription drug prices are to drop, he said, the rules for approving those complex generic drugs need to be rewritten.
Yes, he is a doctor. He’s also been a patient.
Gottlieb is a cancer survivor, having been successfully treated for Hodgkin’s lymphoma.
That experience prompted Gottlieb to become a cancer policy advisor for the National Coalition for Cancer Survivorship. The coalition, in turn, strongly endorsed his nomination to head the FDA.
In a statement issued when Trump nominated him in mid-March, the coalition said that Gottlieb “understands the human toll cancer takes on individuals and families, during both treatment and long-term survivorship. He is open to a wide range of perspectives, including those of the patients whose lives depend on a strong FDA.”
Gottlieb is a physician. He trained at Mount Sinai School of Medicine in New York and completed his residency in internal medicine at Mount
After graduating from Wesleyan University in Connecticut, Gottlieb worked as a healthcare analyst at the investment bank Alex. Brown & Sons in Baltimore, and then went to medical school.
Between 2003 and 2007, Gottlieb served the Bush administration in a wide range of positions at the FDA and in the Centers for Medicare and Medicaid Services. Among his titles: deputy FDA commissioner for medical and scientific affairs. At the FDA, he helped develop standards for drug cocktails to be used for HIV treatment. He also helped draft strategic plans for U.S. biodefense countermeasures as a member of the White House Biodefense Interagency Working Group.
Since 2007, he has been a venture partner at New Enterprise Associates, focusing on healthcare and medical devices investments, and a resident fellow at the American Enterprise Institute, a conservative think tank. He’s also been a clinical assistant professor at New York University School of Medicine and practiced medicine as an attending physician at Stamford Hospital in Connecticut.
He has said he'd divest himself of medical company stocks and recuse himself for a year from decisions involving nearly two dozen companies, including Tolero Pharmaceuticals, Daiichi Sankyo Inc. and GlaxoSmithKline.
MORE IN SCIENCE
Speed up drug approvals at FDA? It's already faster than Europe's drug agency
Dying patients want easier access to experimental drugs. Here's why experts say that's bad medicine




