FDA probes accuracy issue with Abbott’s rapid coronavirus test
- Share via
Federal health officials are alerting doctors to a potential accuracy problem with a rapid test for COVID-19 used at thousands of hospitals, clinics and testing sites across the U.S., including the White House.
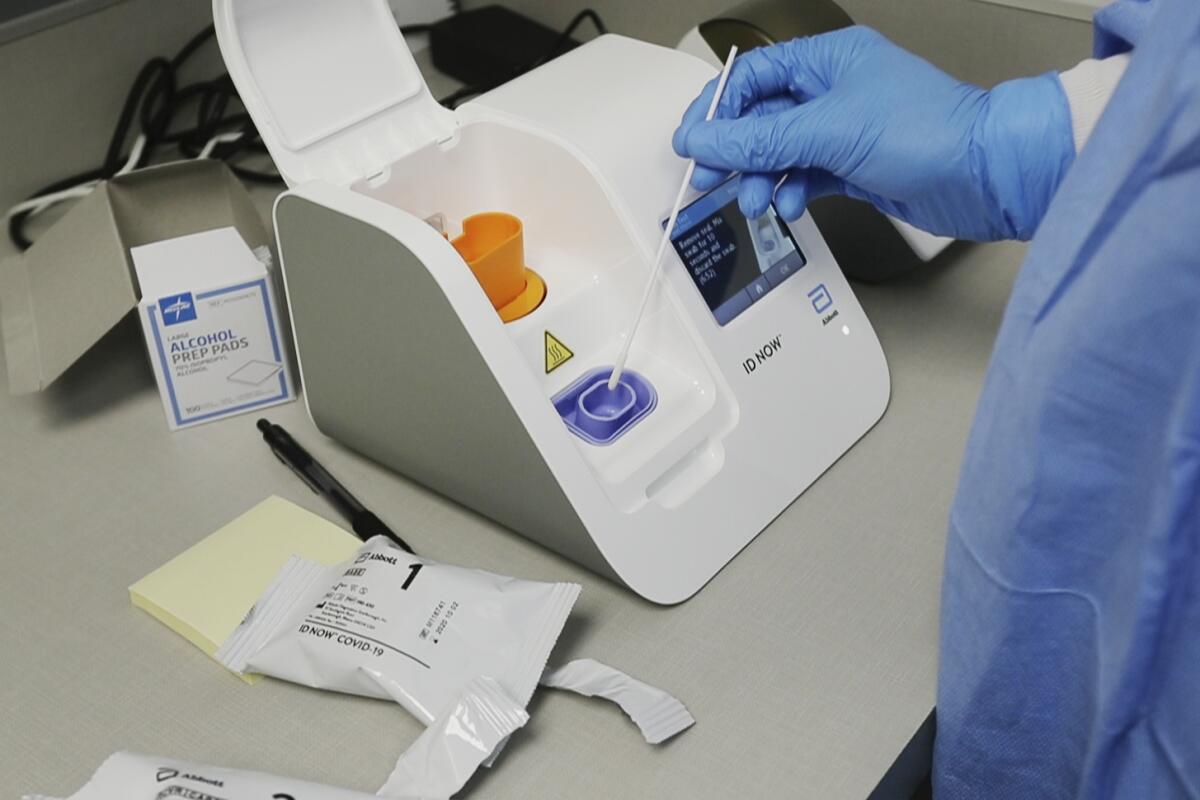
The Food and Drug Administration said late Thursday it is investigating preliminary data suggesting that Abbott Laboratories’ 15-minute test can miss many COVID-19 cases, falsely clearing patients who are actually infected with the coronavirus.
The Abbott test is used daily at the White House to check President Trump and key members of his staff, including those on the coronavirus task force.
The FDA warning came after researchers at New York University reported results suggesting that Abbott’s test can miss up to half the infections detected by a test made by rival Cepheid. The research, which was based on about 100 patients, was posted this week on the BioRxiv website has not been peer-reviewed or published in a medical journal.
The NYU team found that Abbott’s test, run on the company’s portable ID NOW system, missed one-third of the infections caught by Cepheid’s test when swabs were stored in a liquid used to transport laboratory samples. When the samples were kept dry, the test missed 48% of the cases flagged by Cepheid’s test.
The researchers called the test’s performance “unacceptable” due to the risk of falsely clearing patients who could spread the infection to others.
They also acknowledged some shortcomings of the study, including the time needed to transport patient samples to the device for processing.
Abbott rejected the findings, saying the researchers used the test “in ways that it was not designed to be used.”
“ID NOW is intended to be used near the patient with a direct swab test method,” the company said in a statement. The company pointed to other independent study results that found the test accurately detected 90% or more infections.
It’s slowly getting easier to obtain a coronavirus test in California — following an initial rollout marked by restrictions and shortages.
Last month, Abbott changed the instructions for its test to explicitly warn against putting patient samples in transport chemicals. The update came after researchers at the Cleveland Clinic reported a 15% false negative rate for samples stored in the chemicals.
The FDA said in a statement that it is reviewing the data with Abbott and working on a letter to healthcare providers about potential accuracy issues. The agency said physicians may need to confirm the results of a negative Abbott test if patients have signs and symptoms of the virus.
“This test can still be used and can correctly identify many positive cases in minutes,” said Dr. Tim Stenzel, FDA’s diagnostics director.
Regulators also said they are requiring Abbott to conduct follow-up studies on the test’s accuracy.
Stunning visualizations show what the novel coronavirus, SARS-CoV-2, looks like at an atomic level of detail.
The FDA first cleared Abbott’s test in late March under emergency powers used to quickly accelerate the review of tests and treatments during a public health crisis. The agency has authorized more than 90 tests, effectively flooding the market with testing options amid persistent problems ramping up testing nationally.
For weeks, Trump has promoted Abbott’s test as a “game changer.” On Monday, the company’s ID NOW system and test cartridges were displayed prominently at a Rose Garden news conference meant to highlight the nation’s progress on testing.
“These tests are highly sophisticated — very quick, very good,” Trump told reporters.
The federal government has shipped more than 235,000 of the tests to state public health laboratories. And Abbott has separately said it has distributed 1.8 million tests to healthcare facilities in all 50 states.
A White House representative did not immediately respond to a request for comment Thursday night.







