
- Share via
Early in the pandemic, Chris Parks needed a gene and $1 million.
Commuting from his home in Boonton, N.J., to his laboratory in Brooklyn, N.Y., he tuned to the local news. Death counts seemed to have replaced traffic reports. More people were dying of COVID-19 in all five boroughs of New York City.
When he reached his office at IAVI, once known as the International AIDS Vaccine Initiative, on the eighth floor of the Brooklyn Army Terminal, his fellow scientists wanted to know: Would they be working on a COVID-19 vaccine?
Parks didn’t have an immediate answer. He needed a go-ahead from the senior leadership.
And the $1 million — just to get started.
Developing a vaccine is a gamble. Efforts fall short. A virus can disappear.

But Parks knew this was different. The December outbreak in Wuhan, China, alarmed him. Then came news from Thailand, Japan, Korea — and Seattle. One day he pulled out an old textbook, “Fields Virology,” as if he needed to be reminded of where this was headed.
The World Health Organization issued warnings. Experts said hundreds of thousands of Americans would die within a year. More than science, this was becoming a moral responsibility. His inbox filled with questions.
Then one day in mid-February, he got the OK. If the company’s technical expert believed the lab could make a vaccine, then it should try, even if that meant spending its own money.
IAVI’s Brooklyn lab had fewer than 25 scientists. It was tiny compared with Pfizer, Sanofi, Johnson & Johnson, AstraZeneca and Merck, whose scientific staffs numbered in the hundreds.
And IAVI was a nonprofit, an underdog in a high-stakes race.
::
When Parks told his principal scientist, Maoli Yuan, she recognized this historic moment.
IAVI‘s effort was one of about 200 initiatives around the world hunting for a coronavirus killer, and Yuan joined thousands of scientists who put in long hours tracking experiments, following protocols and charting procedures in their cold, sterile laboratories.
Capped, gowned and masked, they worked in anonymity for the good of a world that expected so much of them — and yet knew so little about what they did.
In a series of phone interviews, Parks and Yuan detailed their challenges once they decided to take on the virus.

Parks began with an internet search for a published genome of the novel coronavirus and emailed it to Yuan. Their effort would build upon a scientific revolution that began in the 1970s. Until then, vaccines were developed by either killing a targeted pathogen or reducing it to a less deadly form. Production — growing viruses in chicken eggs, for instance — was slow, and recent outbreaks, such as hepatitis B, demanded a different approach.
Newer vaccine technologies that employ versions of a pathogen’s DNA or RNA use the body’s machinery to replicate and enjoy an economy of speed and scale. Experts believe the pandemic will only accelerate progress.
“This is the golden age of vaccinology,” said Dr. Buddy Creech, director of the vaccine research program at Vanderbilt University Medical Center.
Yuan downloaded the file Parks sent her. It looked like a long run-on sentence, using just four letters — a, t, c and g — repeated 29,882 times. Her job was to create a set of instructions for a biotech company in New Jersey, GenScript, to prepare.
att aaa ggt tta tac ctt ccc agg caa taa
Each letter represents a chemical — adenine, thymine, cytosine, guanine — known as a nucleotide. Nucleotides help select amino acids that combine to form proteins, an essential element for life.
The arrangement of these nucleotides was unique to each gene. The coronavirus had 11. At Yuan’s request, software singled out one, the S gene. This run-on sentence had 3,819 letters and created the S — or spike — protein that the coronavirus needed to infect and replicate.
Working with special software and a set of customized instructions that Parks had developed over the years, Yuan began rearranging the nucleotides. Guanine became adenine, for instance; cytosine became thymine.
She made nearly 950 changes, hoping to modify the coronavirus gene just enough to take the place of a similar gene belonging to a benign virus: the vesicular stomatitis virus, or VSV.
For this to work, she had to find the right balance. If she made too many changes — or too few — the VSV would not survive.
If successful, however, the VSV could carry a portion of the deadly coronavirus along with it like a Trojan horse. Once injected into a person, this Trojan horse would replicate and draw an attack by the immune system.
Once vaccinated, the person would be ready for future invasions.
::
Parks had built a career on this Trojan horse. For almost 15 years, he had been studying VSV as a possible weapon in a fight against HIV and deadly viral fevers.
When Merck turned to VSV for its successful Ebola vaccine five years ago, he knew he was on the right track.
Two additional labs were using VSV for coronavirus vaccines, and nearly 50 others were modifying other viruses — adenovirus, measles, flu — for their Trojan horses. (ImmunityBio, based in El Segundo, is among the labs developing a vaccine by modifying an adenovirus. The firm’s chairman and chief executive is Dr. Patrick Soon-Shiong, who also owns The Times.)
Parks’ research had not yet produced a vaccine, but scientific progress did not always require a breakthrough. Small steps counted as well.
Yuan spent a week analyzing her sequences. Parks studied the literature.
On Feb. 21, Yuan was ready. She emailed her order to GenScript. It would follow her blueprint, build her Trojan horse and deliver it as DNA.
Four weeks later, she and Parks had received nothing.

Had the pandemic disrupted supply chains? Had Yuan made an error rearranging the nucleotides? She began waking up at 4 a.m.
Parks grew restless. Outside his office window, he could see the Statue of Liberty across New York Harbor and the Freedom Tower in the distance. Below him, ferries to Manhattan came and went, but the commuter parking lot was empty. The stillness of the streets reminded him of a science fiction novel.
::
But this was virology, not science fiction. Parks, 61, had grown up in western New Jersey. His family’s working-class roots — his dad was a plumber — meant going to a community college, where he first learned about viruses.
Interest became an obsession. It took him to Rutgers, Penn State and Princeton.
His first job was at Wyeth Pharmaceuticals, where in the early 2000s, he studied VSV — the Trojan horse. It could play a role in developing vaccines to fight HIV and a respiratory virus called RSV.
By 2007, however, Parks felt the landscape change. Wyeth, which would soon be purchased by Pfizer, was shifting its research agenda away from HIV, he said, toward a vaccine for a serious bacterial infection.
The decision for Parks to join IAVI, which was committed to fight HIV, was easy.
Yuan, in her 40s, had grown up in Shanghai.
Her mother was a high school biology teacher, and Yuan was drawn to public health. She married, and her husband was invited to study molecular biology at a medical center in Brooklyn. They moved to America.
She had gone back to visit her parents last year, but she canceled an August trip because of the pandemic. A hunt for a vaccine was personal.
Nor was she alone. Some colleagues lived with parents. Some had medical conditions. Others needed to travel. They accepted the sacrifice that fighting the pandemic required: hours spent between the known and unknown nature of the virus, coaxing from a microscopic world answers to questions that had never been asked.
Few people understood what they did. The experiments were complicated, the monitoring tedious, the science far removed from microscopes and petri dishes of high school. Worse, vaccines had become controversial. Even political. Some efforts drew hostility.
Still the world waited, waited for them to outwit the coronavirus and put things right again, if they could.
“Science without failures is not science,” said John Rose, who has studied viruses since the 1970s and is director of the molecular virology program at Yale School of Medicine, and the coronavirus “is a hard target.”
::
At IAVI, scientists and research associates doubled their workload. Social distancing protocols meant staggered shifts. Personnel were reassigned and lab space reconfigured.
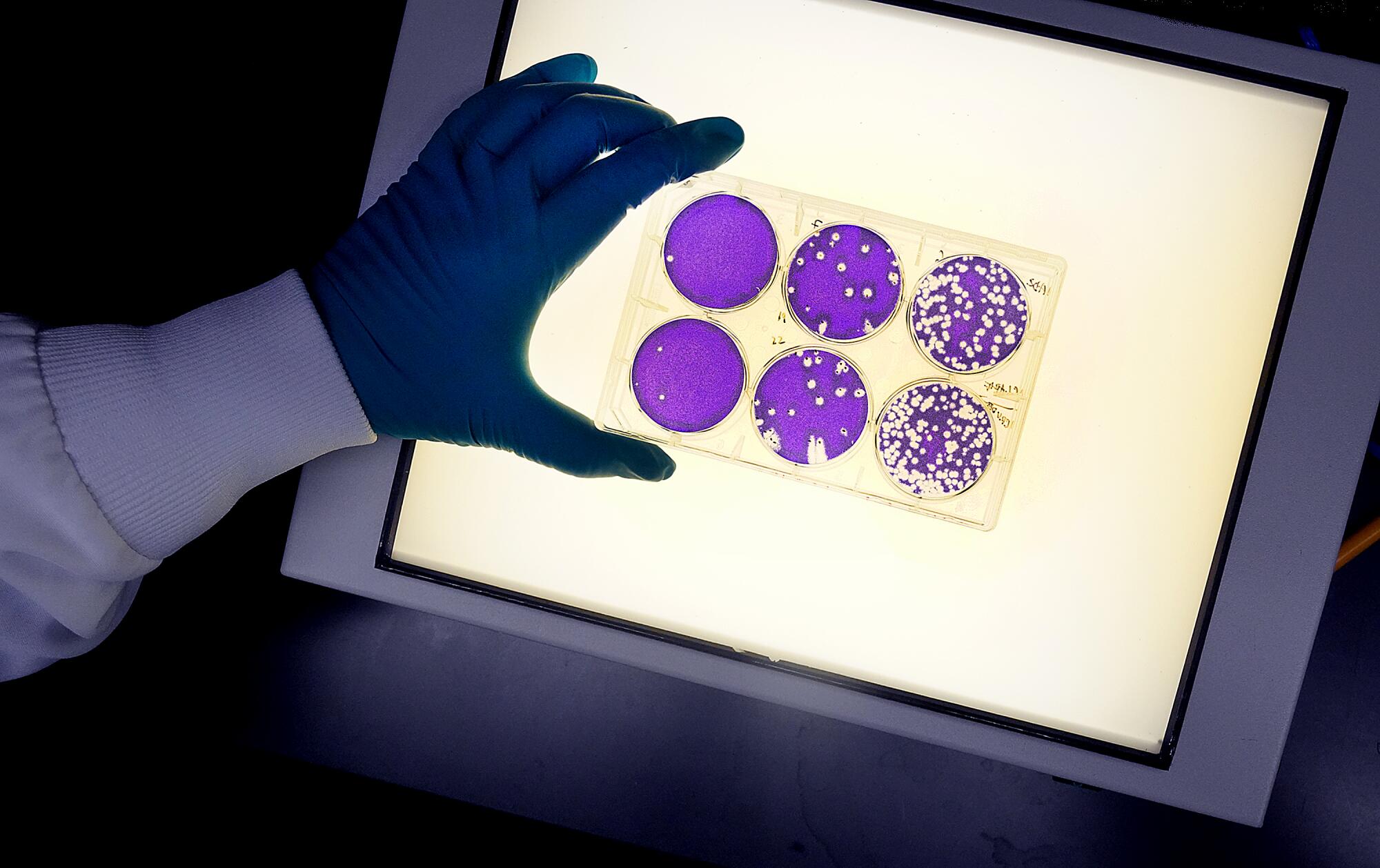
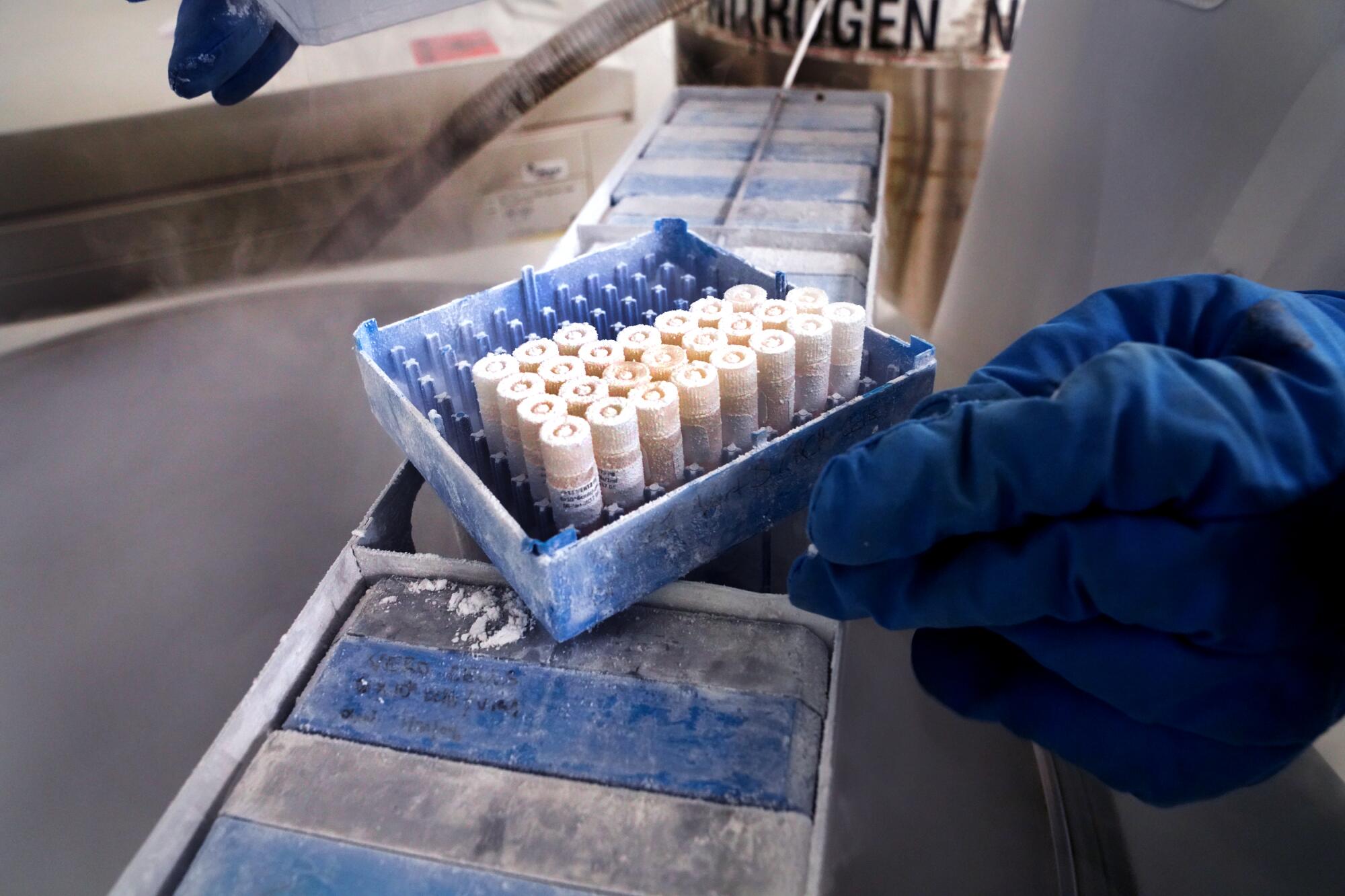
In the so-called cold room, senior research associate Lucia Reiserova put on blue, insulated elbow-length gloves and opened a freezer. She waited for vapors of liquid nitrogen to clear.
Reiserova reached in and pulled out a rack of seven boxes. Each contained 10 vials. She took one and closed the freezer. She removed the thick gloves. Underneath she wore thinner gloves, and the vial felt colder than an ice cube in the palm of her hand.

Inside were cells of African green monkeys. Investigated by Japanese researchers in 1962, these cells, called Vero cells, grew on the outside of the monkeys’ kidneys. The cells lay flat, making invaders conspicuous. They also had no defenses that would inhibit growth of the invaders.
Swapping the vial from hand to hand, Reiserova took it to a warm-water tank and placed it inside. The monkey cells thawed.
Yuan put them into a flask containing nutrients. In 24 hours, their volume doubled.
One week later, on April 1, FedEx made a morning delivery. Yuan opened the envelope and took out a small plastic vial, cool from an ice pack.
She lifted the vial to an overhead light. A clear solution pooled in the base.
Inside the solution was the DNA of VSV with the modified coronavirus S gene that GenScript had prepared.
The Trojan horse.
::
Now would it work? Would it grow and replicate?
The day before, Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, and Dr. Deborah Birx, coordinator of the White House Coronavirus Task Force, had said COVID-19 could kill 240,000 Americans.
New York City was the epicenter.
“We were doing everything as fast as possible,” Parks said. “We knew it was a marathon, but we had to sprint. People were dying.”
Yuan said a prayer. Five weeks of waiting was about to culminate in one hour of work.
She withdrew 10 microliters — about one-fifth of an eyedropper — from the vial containing the Trojan horse and put them into a culture flask along with a special preparation of the monkey cells.
She shook the mixture slightly.
Then she applied a quick burst of electricity that opened pores in the membranes of the cells.
The Trojan horse slipped inside.
Yuan transferred the flask into an incubator and watched it carefully.
She and Parks hoped for a massive infection of the monkey cells within a few days. If this step failed, IAVI would not have a vaccine.
When Parks got home at night, he tried to relax. Taking his wife out for dinner was no longer an option, nor was an occasional Mets baseball or Devils ice hockey game. So he turned to yardwork.
Each day at 5 a.m., he drove the empty streets and across the Verrazano-Narrows Bridge into Brooklyn. He was often first in the lab. Freezers hummed; fans whooshed from ventilated cabinets.
Gowned and protected by goggles and a face mask, he went to the incubator and pulled out the flask.
He held it to a fluorescent light.
Nothing.
::
I see something, but honestly I’m not sure.
Yuan had come into the lab late on a Saturday night. She emailed Parks and took a photograph.
The next morning, he replied. Doesn’t look like much.
Yuan agreed. Eleven hours later, she was back in the lab.
Still not much going on.
After five days, the VSV was languishing. The Trojan horse hadn’t worked.
“How are you going to fix this?” asked senior leadership.

By then, Merck was looking at IAVI’s research. The pharmaceutical company had yet to make a public commitment to a vaccine but wanted to collaborate. It had already begun to share the cost of IAVI’s efforts, and its scientists offered suggestions.
Parks tried to keep his team upbeat.
Yuan could take a new approach, rearrange the nucleotides another way.
Outside, COVID-19 cases were exceeding 1 million worldwide, and 10 million Americans had filed for unemployment.
::
Parks began each day with a ritual.
In the low drone of the lab, he put on personal protective equipment, opened the incubator, reached for the flask with the monkey cells and held them up to the light.
One morning — after nearly two weeks — there they were: comet tails.
The virus was breaking out of the cells and creating trails, like shooting stars.
Damn! Parks thought. We can do this.
He took the flask to a microscope.
In sharper detail, he saw the strange beauty of the VSV destroying its host.
“This was a huge moment,” he said, remembering the details. “We thought it was technically feasible. But was it really?”
He emailed Yuan. The cell fusion is crazy this morning.
She hurried to the lab.
Their original Trojan horse had worked after all.
Viruses evolve, Parks thought. In new environments, they mutate, and perhaps the most difficult practice in laboratory work is letting nature take its course.
“VSV is smarter than I thought,” he said.
::
During the last week of May, Merck said it was joining with IAVI. Operation Warp Speed, the public-private partnership effort to develop a COVID-19 vaccine, included Merck among seven companies leading the race, and Merck received $38 million in federal funding.
Its vaccine, now known as rVSVΔG-SARS-CoV-2, was sent to a lab in Pennsylvania, where it was injected into mice.

After mice, it was injected into hamsters. Lab staff watched for changes in behavior and fluctuations in weight. They collected blood and mucosal samples. Lasers counted T-cells, and a biochemical assay confirmed the presence of coronavirus antibodies.
Weeks later, the hamsters were shipped to a facility where they were exposed to the coronavirus.
Parks read reports: The vaccinated animals showed no signs of respiratory distress, no weight loss.
Parks, Yuan and the IAVI team celebrated.
Rhesus macaques were next. Testing started soon after the Fourth of July weekend.
Four months later, on Nov. 2, the vaccine — or a placebo — was shot into the deltoid muscle of a healthy adult, signaling the start of the Phase 1 clinical trial.
Parks is optimistic. But there are no guarantees.
Two other vaccine initiatives, well ahead in their research, briefly stopped testing after a few volunteers became sick, and on Monday, the day that Pfizer announced its vaccine was more than 90% effective, Brazil suspended a trial of a Chinese vaccine, citing “an adverse, serious event.”
“I’ve been around the business so long that I know things can go wrong,” Parks said. “So much of science turns out negative. Positive results are rare.”
As more people around the world became exposed to the coronavirus, however, he knew that more than one vaccine would be needed.
And the underdog had entered the race.









