Indonesia is first to greenlight Novavax COVID-19 vaccine
- Share via
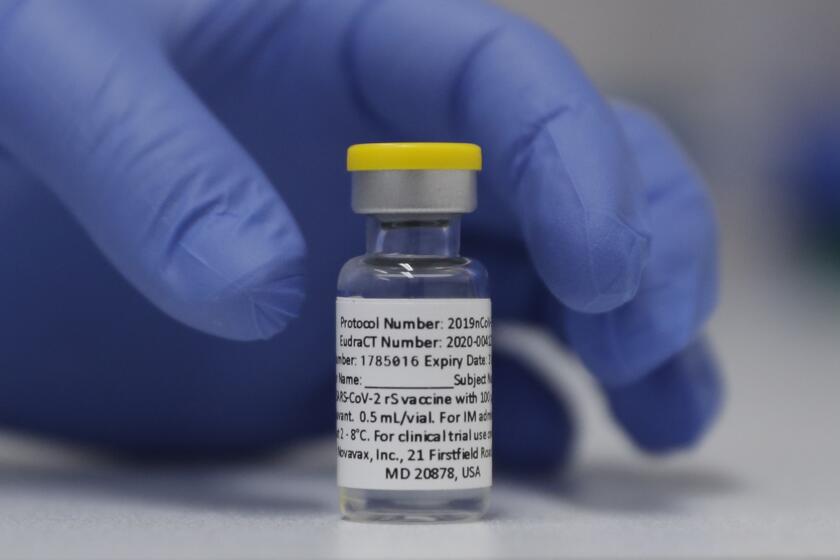
JAKARTA, Indonesia — Biotechnology company Novavax said Monday that Indonesia has given the world’s first emergency use authorization for its COVID-19 vaccine, which uses a different technology than the current shots.
The vaccine is easier to store and transport than some of the alternatives, which could enable it to play an important role in bolstering supplies in poorer countries around the world.
The two-dose Novavax vaccine is made with lab-grown copies of the spike protein that coats the coronavirus surface. That’s very different from the widely used mRNA vaccines made by Pfizer-BioNTech and Moderna that deliver genetic instructions for the body to make copies of the spike protein itself.
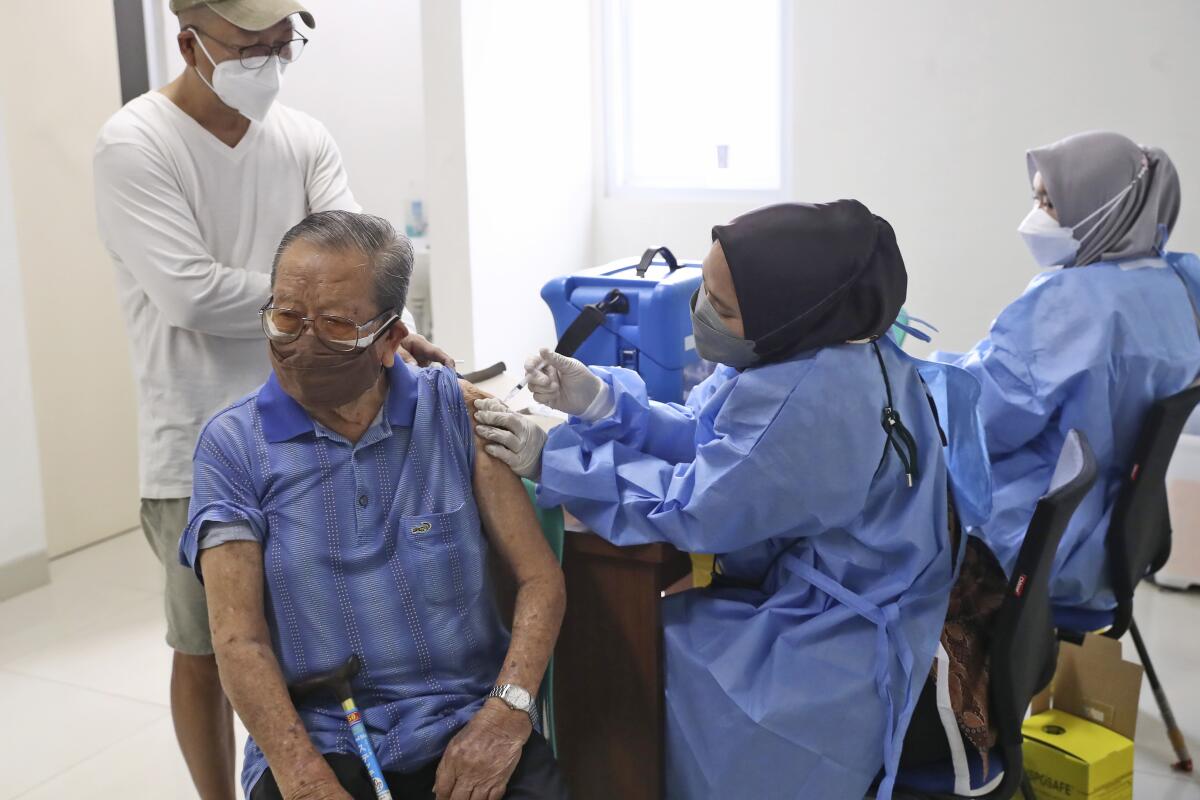
The emergency authorization of the vaccine is a “very important step” for Indonesia’s COVID-19 vaccination program, said Indonesian epidemiologist Dicky Budiman.
“This vaccine will be much easier to transport, store and distribute in a place like Indonesia, where we have many islands,” he said.
Budiman said if the rollout of the vaccine is successful, that could lead to its approval and use in other developing nations.
The need for more vaccines remains critical in many countries, including Indonesia.
In June, U.S.-based Novavax announced the vaccine had proved about 90% effective at preventing cases of COVID-19 in a study of nearly 30,000 people in the U.S. and Mexico. It also worked against variants circulating in those countries at the time, it said.
Vaccine maker Novavax says its shot is highly effective against COVID-19 and also protects against variants.
The company said side effects were mild and included tenderness at the injection site, headache, aches and pains and fatigue.
In October, it addressed concerns that production of the vaccine had been slowed by a lack of raw materials and other issues, saying it planned to “achieve a capacity of 150 million doses per month by the end of the fourth quarter” through partnerships with Serum Institute of India, SK Bioscience in South Korea and Takeda in Japan, among others.
Novavax said it has already filed for authorization of the vaccine in the United Kingdom, the European Union, Canada, Australia, India and the Philippines.
Indonesia was battered by a deadly wave of COVID-19 fueled by the Delta variant and post-holiday travel from June through August. The number of new cases has now dropped, averaging less than 1,000 a day since mid-October.
Why the question of vaccinating younger children against COVID-19 is more complicated than it would initially appear.
About 36% of people in Indonesia have received two doses of a vaccine, and about 58% have received one dose, according to the Ministry of Health.
More than 143,400 people have died from the virus in Indonesia. The number is thought to be an undercount due to low testing and contact tracing.
Associated Press writer Niniek Karmini contributed to this report.