Pfizer’s COVID shots appear 73% effective in children under 5
- Share via
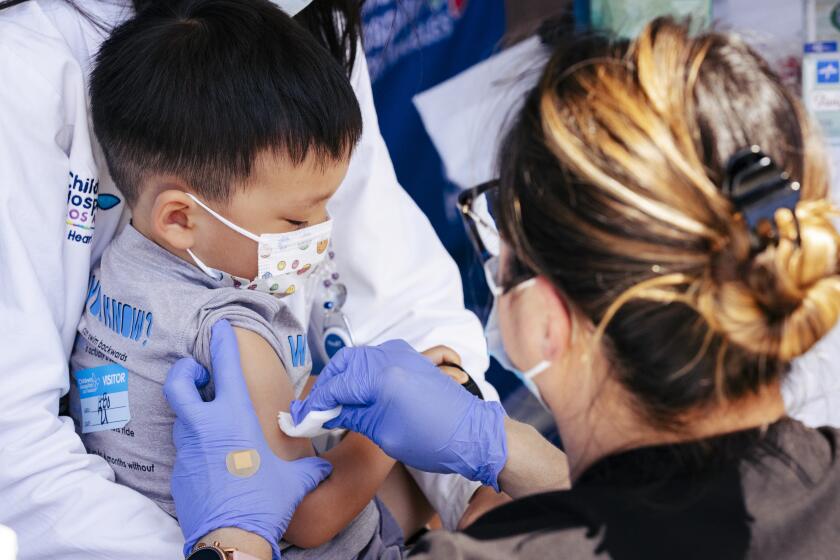
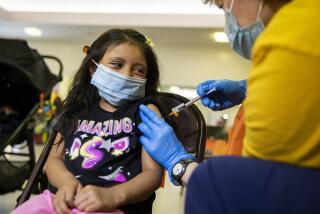
Pfizer’s COVID-19 vaccine was 73% effective in protecting children younger than 5 as Omicron spread in the spring, the company announced Tuesday.
Vaccinations for babies, toddlers and preschoolers opened in the U.S. in June after months of delay. Only about 6% of youngsters ages 6 months through 4 years had gotten at least one dose of a COVID-19 vaccine by mid-August, according to the American Academy of Pediatrics.
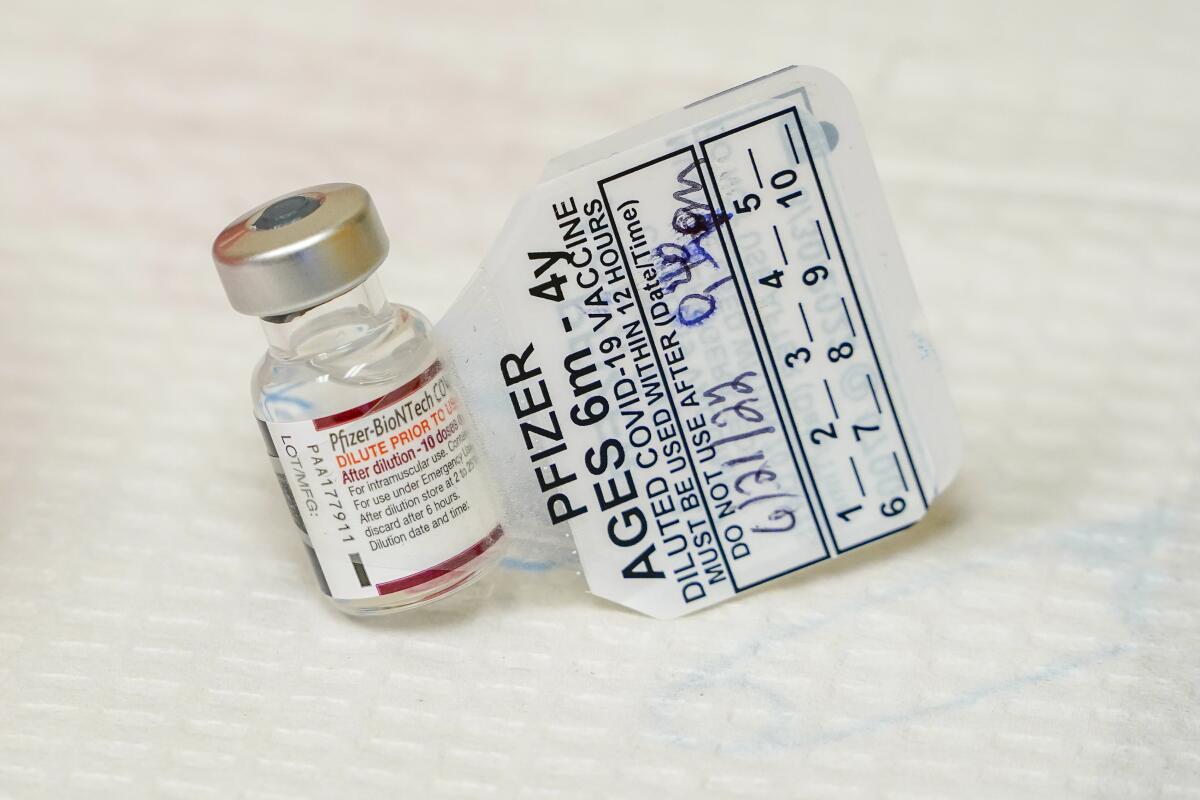
Health authorities authorized the smaller vaccine doses made by Pfizer and its partner BioNTech based on a study showing they were safe and produced high levels of virus-fighting antibodies. But there was only preliminary data on how that translated into effectiveness against symptomatic COVID-19.
The new update analyzed COVID-19 diagnoses between March and June in Pfizer’s ongoing study of the three-dose vaccine. There were 21 COVID-19 cases among the 351 children under 5 who got dummy shots, compared to just 13 among the 794 youngsters given three vaccine doses.
Both Pfizer and Moderna are considered safe for kids of all ages, but here are a couple of differences that parents can consider.
The infections primarily were caused by the BA.2 Omicron subvariant that was circulating at the time. Today, another Omicron relative, BA.5, is causing most infections in the U.S. and much of the world.
In older children and adults, the COVID-19 vaccines have been used long enough to prove that they remain strongly protective against severe disease and death even as the coronavirus mutates.
Pfizer this week asked U.S. regulators to authorize a modified version of its vaccine that targets the BA.5 and BA.4 strains, to be used as boosters for people 12 and older this fall. The company said it also is developing updated shots for children under 12.