- Share via
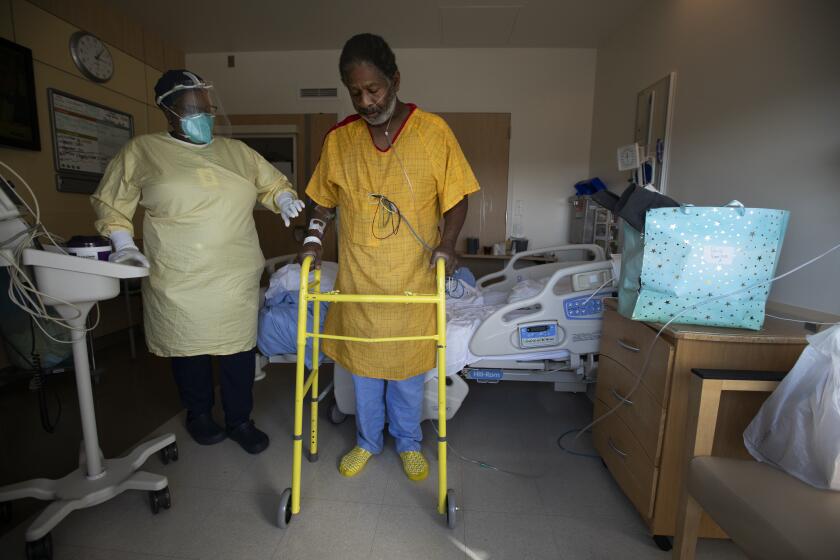
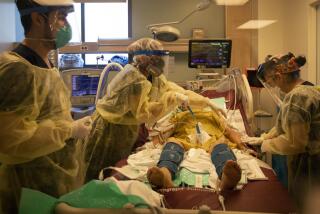
Roots Community Health Center was slammed in 2020, with lines for its COVID-19 testing stations stretching around the block and exam rooms full of people struggling to breathe.
Patient after patient at the East Oakland clinic extended their fingers so that healthcare workers could clip on a pulse oximeter, a device that measures the degree to which red blood cells are saturated with oxygen. For healthy people, a normal “pulse ox” reading is typically between 95% and 100%.
The Centers for Disease Control and Prevention had instructed providers to give oxygen therapy to any COVID patient with a pulse oximeter reading below 90%. Like their counterparts around the country, Roots doctors advised concerned patients to buy inexpensive pulse oximeters so they could monitor their levels at home.
As the pandemic ground on, it became clear that Black and brown patients were dying of COVID at disproportionately high rates, both across the U.S. and in Roots’ own Alameda County.
In the rare hour when she wasn’t in the clinic, Roots founder and Chief Executive Dr. Noha Aboelata paged through medical research in search of answers that might help her patients, the vast majority of whom were Black or brown.
One paper in the New England Journal of Medicine stopped her cold. University of Michigan researchers examined records of thousands of hospitalized COVID patients and looked for instances of “occult hypoxia” — a situation when a patient’s pulse oximeter reads in the healthy range, but their actual blood oxygen levels are dangerously low. The researchers found that this happened to Black patients nearly three times as often as it did to white patients.

Aboelata recalled the “devastating feeling” of diving further into the literature and realizing that this disparity was not a new discovery.
Research dating back to 1990 found that inaccurate pulse oximeter readings were more common in Black patients than non-Black ones. In 2005, detailed lab experiments showed that pulse oximeters frequently overestimated blood oxygen levels in patients with more skin pigmentation.
“This device is really used almost like a vital sign, like you would use a blood pressure cuff,” Aboelata recalled. “How horrified you would feel if you suddenly found out that your blood pressure cuff didn’t work on a certain demographic of your patients?”
She alerted colleagues to the findings and investigated the effect the devices had on the fates of COVID patients of color. She asked the Food and Drug Administration to require pulse oximeter makers to test their devices on people of color and to warn consumers about the heightened risk of false readings. Attorneys for Roots sent letters to companies that made or sold pulse oximeters in California asking them to improve their products and disclose their limitations.
One of the hallmarks of the COVID-19 pandemic in the United States is that it disproportionately strikes people of color. But it doesn’t have to be that way.
When little changed, Roots filed a lawsuit in November against CVS, Walgreens, GE Healthcare and nine other companies that make, sell or distribute pulse oximeters in California.
“The pigmentation-derived inaccuracies of pulse oximeter readings in people with darker skin consistently skew — or are biased — in one dangerous direction: showing that their blood is more oxygenated than it is in reality,” the lawsuit states. “Individuals with darker skin who use the devices are no less entitled to accurate readings than individuals with lighter skin.”
The suit asks that the companies either find a fix or place warning labels on the products to alert users that skin pigment may affect results.
Before pulse oximeters were widely adopted in the 1980s, the only way to gauge a patient’s blood oxygen saturation was to draw a sample of blood from their arterial vein, a painful procedure that had to be followed by immediate laboratory analysis. The portable, noninvasive oximeters were “a true innovation,” said Dr. Phil Bickler, a neuroanesthesiologist who directs the Hypoxia Research Laboratory at UC San Francisco.
“It’s arguably one of the most important clinical monitors ever devised,” Bickler said, second only to the thermometer.

A pulse oximeter works by shining a light that passes through skin, blood and tissues in the finger and then measuring how much light comes out the other side.
Oxygen-rich blood absorbs more infrared light. So does melanin, the pigment that helps determine skin, hair and eye color. As a result, patients with darker skin tones are more likely to get pulse oximeter readings that show their blood oxygen saturation to be higher than it actually is.
Skin pigment isn’t the only variable that can skew those results. Cold hands, trembling fingers, incorrect probe placement, even nail polish can throw a reading off by a few percentage points too. Knowing this, doctors traditionally used the pulse ox as one data point among many when determining a patient’s course of treatment.
Then COVID-19 hit. As emergency rooms filled and oxygen tanks grew scarce, the CDC anointed pulse oximeter readings as the official standard in its guidelines for COVID care: Below 90%, the patient should be started on oxygen therapy. Above that, it was the doctor’s call.
As the sheer volume of patients grew, so did the number of people with occult hypoxia. Their pulse ox readings were 92% or higher, yet they often had shortness of breath, erratic heartbeats, headaches, confusion and other symptoms of low oxygen saturation.
Many providers around the country also noted that patients with occult hypoxia were more likely to have darker-toned skin.
“Honestly, we had no idea what to make of it,” said Dr. Michael Sjoding, a pulmonologist at the University of Michigan.
Richard Perry, who by his own sheer grit built a middle-class life for his family in Compton, was waging battle on two fronts: Fighting against COVID-19, and trying desperately to hold on to everything he had worked for.
He and his colleagues initially wondered whether something about the SARS-CoV-2 virus itself made it harder to detect hypoxia.
Then Sjoding came across an article by Amy Moran-Thomas, a medical anthropologist at MIT. After spending sleepless nights monitoring her husband’s pulse oximeter readings as he suffered through COVID, Moran-Thomas began digging into the history of the device.
She found the 1990 paperthat noted hypoxic Black patients were more likely to get deceptively high readings. She found the 2005 study from Bickler’s lab noting the devices were more likely to overestimate oxygen saturation in patients with dark skin than in those with light skin, results the lab confirmed in a follow-up study two years later.
“I was shocked, because I’m a pulmonary critical care physician, I’m a lung doctor, and I didn’t know this whole literature,” Sjoding said.
He and his colleagues pulled data from their own hospital and found Black patients had nearly three times the rate of occult hypoxia as white patients. They published their results in December 2020.
After Aboelata read their paper, she scoured her memory for patients the devices might have betrayed.
She recalled a Black man she had tried to get approved for home oxygen therapy prior to the pandemic. Medicare only paid for the treatment if a patient’s oxygen saturation was below 90%, and “his pulse ox reading just looked too good compared to what I was seeing,” Aboelata said. She sent him to the hospital for an arterial blood gas draw. Sure enough, his oxygen was low enough to qualify.
Patients shared similar stories, “things like, ‘The ambulance didn’t take them to the hospital because they said that their reading was fine,’ or, ‘We were sent home from the emergency department because they said our reading was fine,’” Aboelata said.
In normal times, she said, providers are much more likely to err on the side of caution for a potentially hypoxic patient. But in the worst days of COVID, every bed, oxygen tank and minute was precious. Providers relied on what they believed was the pulse oximeter’s impartial measure to make extremely difficult decisions, unaware that the device did not evaluate all patients equally well.
Aboelata and colleagues from UCSF and Sutter Health’s Institute for Advancing Health Equity published their own study in the American Journal of Epidemiology showing that Black patients whose pulse oximeters overestimated blood oxygen levels waited an extra 4½ hours, on average, to start supplemental oxygen. They were also slightly less likely to be admitted to the hospital or receive oxygen therapy at all.
“There’s just no way to really know how far-reaching this impact is,” Aboelata said. “The likelihood [is] that people were left home to die, or sent home to die.”
The devices that measure blood oxygen levels get a closer look from regulators after studies suggest they don’t work as well for patients of color.
In February 2021, the FDA issued a safety notice cautioning users that pulse oximeters can be thrown off by a number of variables, including skin pigment.
The following year, the FDA convened an advisory committee on the topic. The panel recommended the agency demand better consumer labels and more stringent testing from companies seeking approval for their devices.
Currently, the FDA recommends — but doesn’t require — that pulse oximeter makers ensure that in their clinical trials, either two participants or 15% of total participants are “darkly pigmented” people, a definition open to interpretation.

This month, the panel advised the FDA to require that new devices be tested on at least 24 people whose skin tones collectively span the Monk Skin Tone scale, a 10-color palette often used to train artificial intelligences to recognize people of different colors. The proposal would divide the scale into three parts, with each part represented by at least 25% of study participants.
To better understand the relationship between skin pigment and pulse ox accuracy, the FDA funded a study at Bickler’s UCSF lab. Results are expected this summer.
“Some companies have posted data showing good performance with darkly pigmented skin for their devices. But I know that those have been tested under ideal conditions,” said Bickler, whose lab investigates the effects of low oxygen on the human body and the devices that measure it. “When pulse oximeters are used in the real world, conditions are not ideal. People are dehydrated, they’re in shock, they’re moving. There’s all kinds of interference that can happen and that get in the way of good performance.”
For Bickler, it’s gratifying to see the government finally address a problem that has been known for decades but that device manufacturers seemed reluctant to address.
“There’s a lot of inertia and denial in the industry,” he said. “It was an inconvenient problem that could be ignored, up until COVID.”

The Times reached out to all the defendants being sued by Roots. Those that responded declined to comment on pending litigation.
Only one company has taken actions to address Roots’ concerns. Illinois-based NuvoMed pulled its pulse oximeters from the market in California and agreed to place warning labels on their remaining inventory after receiving Roots’ October letter, said Jonathan Weissglass, the clinic’s attorney.
“Ideally, we’d like the pulse oximeters to be fixed so that the problem doesn’t occur,” Weissglass said. “In the meantime, we feel there needs to be an adequate warning about the inaccuracies for people with darker skin. ... We’ve all seen warning labels that say, ‘Pregnant women should consult with a doctor before using’ or something like that. It’s the same basic idea.”
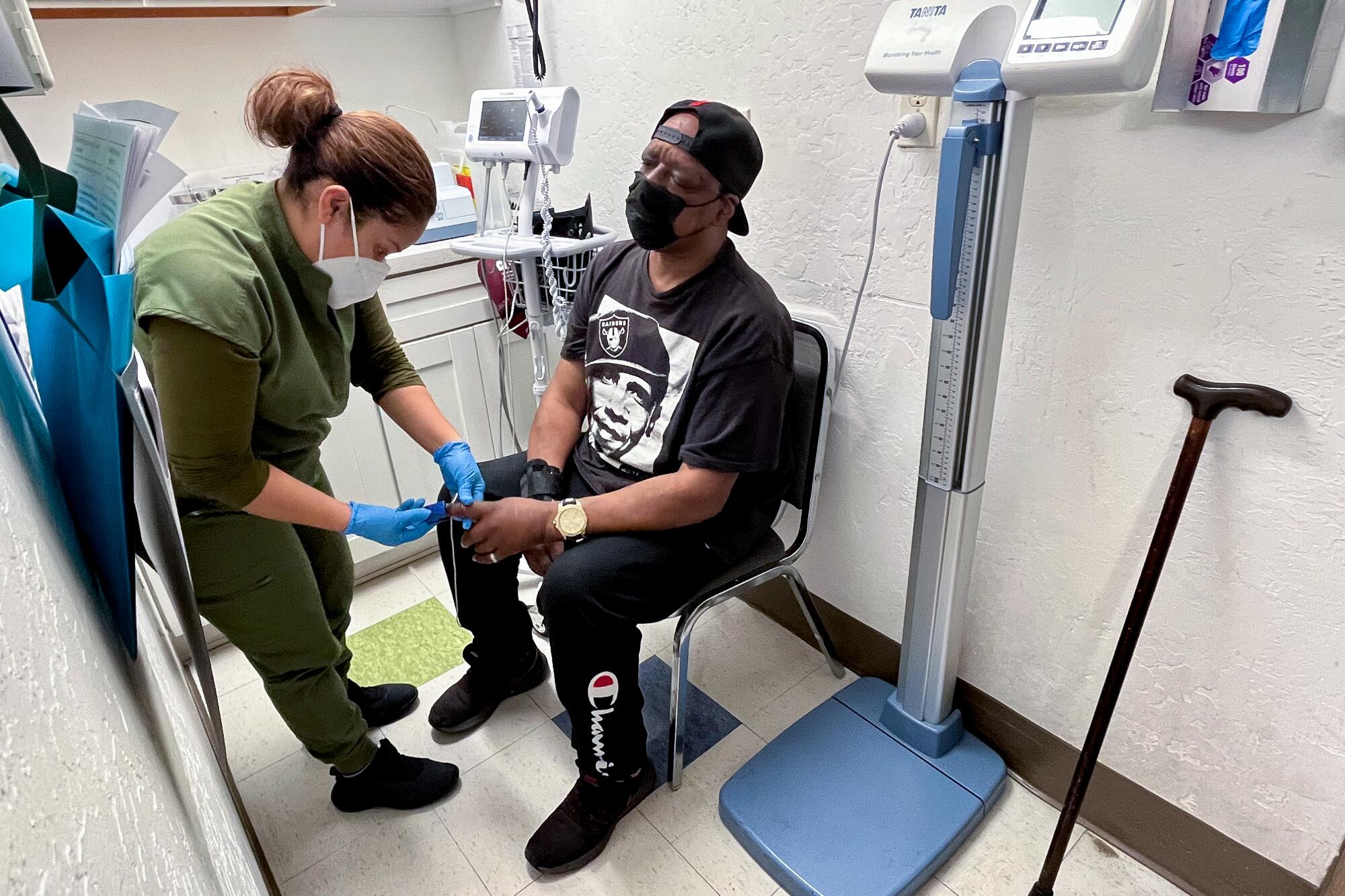
On a recent afternoon at the clinic, medical assistant Evelyn Rivas clipped a pulse oximeter onto Ja-May Scott’s index finger as she checked his vital signs.
The devices are still an important part of Roots’ toolkit. But “we just view it with more suspicion, frankly, in a lot of our patients,” Aboelata said. “We would really like to be equipped with devices that we know can be accurate for all skin tones. And we feel like in 2024, this shouldn’t be too much to ask.”