EU regulator authorizes Moderna’s COVID-19 vaccine for use
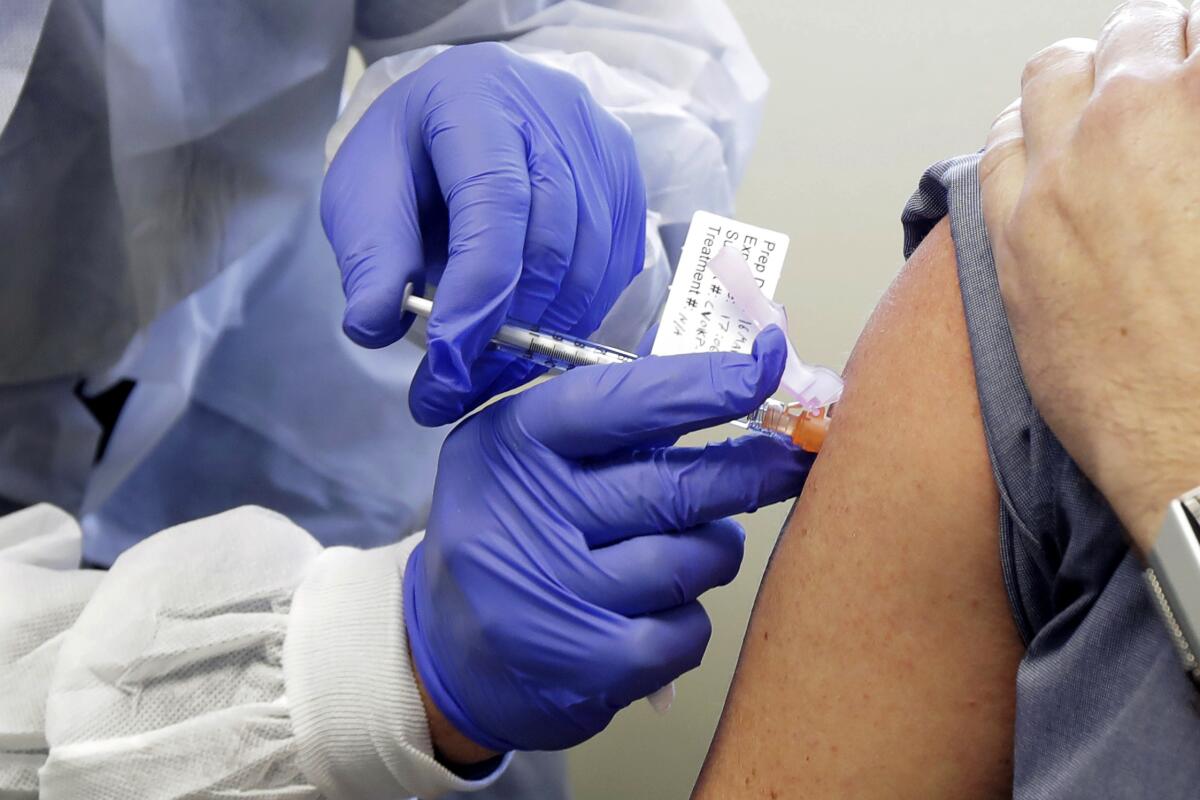
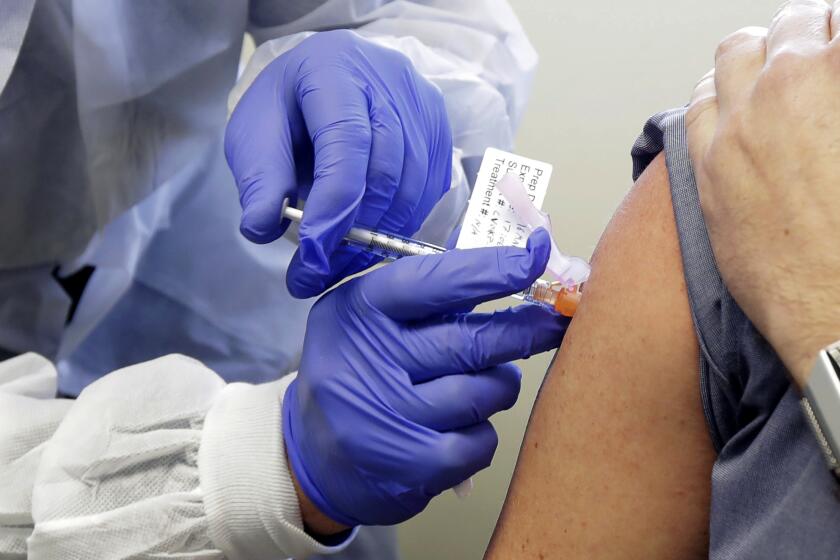
The European Union’s medicines regulator gave the green light Wednesday to Moderna’s COVID-19 shot, a decision that gives the 27-nation bloc a second vaccine to use in the battle to tame the coronavirus as it rampages across the continent.
The approval recommendation by the European Medicines Agency’s human medicines committee — which was later rubber-stamped by the EU’s executive commission — comes amid high rates of infections in many EU countries and strong criticism of the slow pace of vaccinations across the region of some 450 million people.
“This vaccine provides us with another tool to overcome the current emergency,” said Emer Cooke, executive director of the EMA. “It is a testament to the efforts and commitment of all involved that we have this second positive vaccine recommendation just short of a year since the pandemic was declared” by the World Health Organization.
European Commission President Ursula von der Leyen welcomed the approval and added in a tweet: “Now we are working at full speed to approve it & make it available in the EU.”
The EMA has already approved the COVID-19 vaccine made by Pfizer and Germany’s BioNTech. Both the Pfizer-BioNTech vaccine and the Moderna vaccine require giving people two shots.
Ahead of the meeting on the Moderna vaccine, the European regulator said in a tweet that its experts were “working hard to clarify all outstanding issues with the company.” It did not elaborate on what those issues were. Moderna also declined to comment.
Pfizer and Moderna will rake in the big bucks from their COVID vaccines. That’s a sign of a broken system.
The EU regulator gave the green light to use the Moderna vaccine on people 18 and older.
Cook stressed that EU authorities “will closely monitor data on the safety and effectiveness of the vaccine to ensure ongoing protection of the EU public. Our work will always be guided by the scientific evidence and our commitment to safeguard the health of EU citizens.”
Early results of large, still-unfinished studies show that both the Moderna and the Pfizer-BioNTech vaccines appear safe and strongly protective, although Moderna’s is easier to handle since it doesn’t need to be stored at ultra-freezing temperatures.
The U.S., Canada and Israel have already authorized use of the Moderna vaccine. The U.S. gave it the green light for emergency use in people over 18 on Dec. 18, followed by Canada’s interim authorization, also for people over 18, five days later. Israel authorized the vaccine Monday.
When a freezer malfunctioned, a small Ukiah hospital had to use or lose its Moderna COVID dosages quickly, becoming a case study in mass inoculation.
Moderna said Monday that it is increasing its estimate for global vaccine production in 2021 from 500 million to 600 million doses. The company said it is “continuing to invest and add staff to build up to potentially 1 billion doses for 2021.”
Both the Moderna and Pfizer-BioNTech shots are mRNA vaccines, made with a groundbreaking new technology. They don’t contain any coronavirus, meaning that they cannot cause infection. Instead, they use a piece of genetic code that trains the immune system to recognize the spike protein on the surface of the coronavirus and to attack if the real thing comes along.
The EU officially began administering the Pfizer-BioNTech vaccination shots Dec. 27, but the speed of each nation’s inoculation program has varied widely. France vaccinated only about 500 people in the first week, while Germany vaccinated 200,000. The Dutch began vaccinations Wednesday, the last EU nation to start doing so.
German Health Minister Jens Spahn — who has in the past been critical of the slow pace of the EMA — said he expected the Moderna vaccine to begin rolling out to EU nations next week. Germany would get 2 million doses in the first quarter and 50 million in all of 2021, Spahn told reporters in Berlin.
“The problem is the shortage of production capacity with global demand,” he said.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.