CDC supports expansion of COVID-19 booster rollout and mixing shots
- Share via
WASHINGTON — Millions more Americans can get a COVID-19 booster and may choose a different company’s vaccine for that next shot, federal health officials said Thursday.
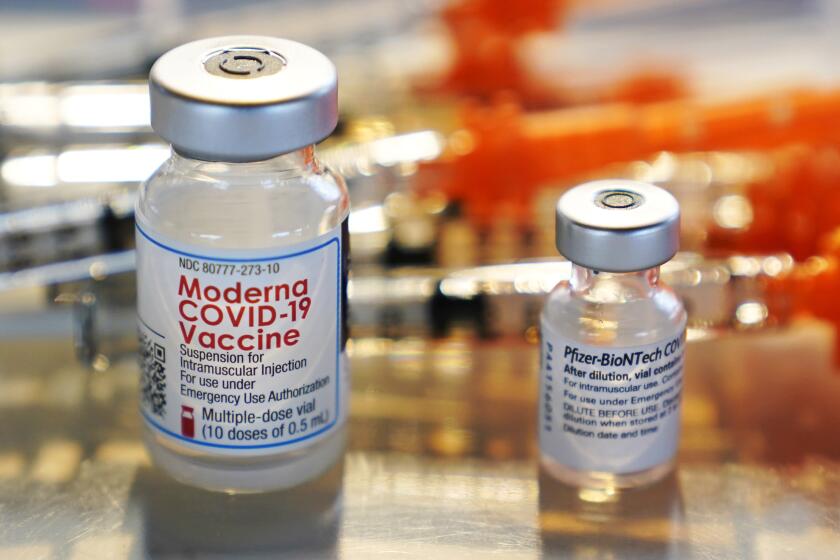
Certain people who received Pfizer vaccinations months ago already are eligible for a booster, and now the Centers for Disease Control and Prevention says specific Moderna and Johnson & Johnson recipients qualify, too.
And in a bigger change, the agency is allowing the flexibility of “mixing and matching” that extra dose regardless of which type people received first.
The Food and Drug Administration authorized such an expansion of the nation’s booster campaign on Wednesday, and on Thursday a CDC advisory panel endorsed the move.
CDC Director Rochelle Walensky had the final word on who gets the extra doses.
“These past 20 months have taught us many things, but mostly to have humility,” she told the panel. “We are constantly learning about this virus, growing the evidence base and accumulating more data.”
There still are restrictions on who qualifies for a booster, and when. Starting six months after a second Pfizer or Moderna vaccination, people are urged to get a booster if they’re 65 or older, live in a nursing home, or are at least 50 and at increased risk of severe disease due to health problems.
Scientists are trying to decide who should be eligible for COVID-19 booster shots, but they’re missing key data that would help them.
Boosters also were allowed, but not urged, for adults of any age at increased risk of infection because of health problems, their jobs or their living conditions. That includes healthcare workers, teachers and people in jails or homeless shelters.
Moderna’s booster will come at half the dose of the original two shots.
As for recipients of the single-shot J&J vaccine, a COVID-19 booster is recommended for everyone at least two months after their vaccination. That’s because the J&J vaccine hasn’t proved as protective as the two-dose Moderna and Pfizer options.
The CDC panel didn’t explicitly recommend anyone get a different brand than they started with, but left open the option — saying only that a booster of some sort was recommended.
However, some of the advisors said they would prefer that J&J recipients receive a competitor’s booster, citing preliminary data from an ongoing government study that suggested a bigger boost in virus-fighting antibodies from that combination.
“We’re at a different place in the pandemic than we were earlier,” when supply constraints meant people had to take whatever shot they were offered, noted CDC advisor Helen Keipp Talbot of Vanderbilt University.
She called it “priceless” to be able to choose a different kind for the booster if, for example, someone might be at risk of a rare side effect from a specific vaccine.
COVID-19 boosters are an extra dose of the original vaccine, and some wonder why they weren’t better matched to the Delta variant.
About two-thirds of Americans eligible for COVID-19 shots are fully vaccinated, and the government says getting first shots to the unvaccinated remains the priority.
While health authorities hope boosters will shore up waning immunity against milder coronavirus infections, all of the vaccines offer strong protection against hospitalizations and death, even as the extra-contagious Delta variant burns through the country.
The CDC’s advisors wrestled with whether people who don’t really need boosters should be getting them — especially young, otherwise healthy adults whose only qualification is their job.
Dr. Sarah Long of Drexel University voiced concerns about opening those people to rare but serious side effects from another dose if they are already adequately protected.
“I have my own concerns that we appear to be recommending vaccines for people who I don’t think need it,” agreed Dr. Beth Bell of the University of Washington.
We look at the science behind the need for COVID-19 booster shots.
But Bell stressed that the vaccines work and that moving forward with the recommendations makes sense for the sake of being clear and allowing flexibility when it comes to boosters.
Despite the concerns by some members, the panel’s vote ended up being unanimous.
The vast majority of the nearly 190 million Americans who are fully vaccinated against COVID-19 have received the Pfizer or Moderna options; only about 15 million received the single-dose J&J shot.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.